Abstract
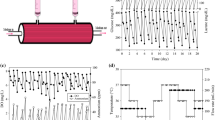
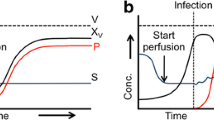
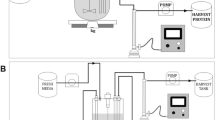
Most bio-industrial mammalian cells are cultured in serum-free media to achieve advantages, such as batch consistency, suspended growth, and simplified purification. The successful development of a serum-free medium could contribute to a reduction in the experimental variation, enhance cell productivity, and facilitate biopharmaceuticals production using the cell culture process. Commercial serum-free media are also becoming more and more popular. However, the cell line secrets its own recombinant product and has special nutritional requirements. How can the composition of the proprietary medium be adjusted to support the specific cell’s metabolism and recombinant protein? This article uses statistical strategies to modify the commercial medium. A design of experiments is adopted to optimize the medium composition for the hybridoma cell in a serum-free condition. The supplements of peptone, ferric citrate, and trace elements were chosen to study their impact on hybridoma growth and antibody production using the response surface methodology. The stimulatory effect of the developed formulation on hybridoma growth was confirmed by the steepest ascent path. The optimal medium stimulated the hybridoma growth and antibody production in three diverse systems: a static plate, an agitated spinner flask, and a hollow fiber reactor. The cells in the developed serum-free medium had a better antibody production as compared to that in the commercial medium in the hollow fiber reactor. Our results demonstrated that the facile optimization for medium and antibody production was successfully accomplished in the hybridoma cells.








Similar content being viewed by others
References
Alcaín FJ, Löw H, Crane FL (1995) Iron at the cell surface controls both DNA synthesis and plasma membrane redox system. Protoplasma 184:233–237
Chabanon G, Alves da Costa L, Farges B, Harscoat C, Chenu S, Goergen JL, Marc A, Marc I, Chevalot I (2008) Influence of the rapeseed protein hydrolysis process on CHO cell growth. Biores Technol 99:7143–7151
Chua F, Oh SKW, Yap M, Teo WK (1994) Enhanced IgG production in eRDF media with and without serum, A comparative study. J Immunol Methods 167:109–119
Coombs MRP, Grant T, Greenshields AL, Arsenault DJ, Holbein BE, Hoskin DW (2015) Inhibitory effect of iron withdrawal by chelation on the growth of human and murine mammary carcinoma and fibrosarcoma cells. Exp Mol Pathol 99:262–270
Coronel J, Klausing S, Heinrich C, Noll T, Figueredo-Cardero A, Castilho LR (2016) Valeric acid supplementation combined to mild hypothermia increases productivity in CHO cell cultivations. Biochem Eng J 114:101–109
Corrêa AL, Senna JPM, de Sousa ÁPB (2016) Effects of passage number on growth and productivity of hybridoma secreting MRSA anti-PBP2a monoclonal antibodies. Cytotechnology 68:419–427
Davami F, Eghbalpour F, Nematollahi L, Barkhordari F, Mahboudi F (2015) Effects of peptone supplementation in different culture media on growth, metabolic pathway and productivity of CHO DG44 Cells; a new insight into amino acid profiles. Iran Biomed J 19:194–205
Dhanasekaran M, Indumathi S, Lissa RP, Harikrishnan R, Rajkumar JS, Sudarsanam D (2013) A comprehensive study on optimization of proliferation and differentiation potency of bone marrow derived mesenchymal stem cells under prolonged culture condition. Cytotechnology 65:187–197
Dong J, Mandenius CF, Lübberstedt M, Urbaniak T, Nüssler AKN, Knobeloch D, Gerlach JC, Zeilinger K (2008) Evaluation and optimization of hepatocyte culture media factors by design of experiments (DoE) methodology. Cytotechnology 57:251–261
Ferreira SL, Santos WND, Quintella CM, Neto BB, Bosque-Sendra JM (2004) Doehlert matrix: a chemometric tool for analytical chemistry—review. Talanta 63:1061–1067
García Münzer DG, Ivarsson M, Usaku C, Habicher T, Soos M, Morbidelli M, Pistikopoulos EN, Mantalaris A (2015) An unstructured model of metabolic and temperature dependent cell cycle arrest in hybridoma batch and fed-batch cultures. Biochem Eng J 93:260–273
Knöspel F, Schindler RK, Lübberstedt M, Petzolt S, Gerlach JC, Zeilinger K (2010) Optimization of a serum-free culture medium for mouse embryonic stem cells using design of experiments (DoE) methodology. Cytotechnology 62:557–571
Kovár J (1988) Hybridoma cultivation in defined serum-free media: growth-supporting substances. V. Trace elements. Folia Biol 34:35–41
Lee J, Tscheliessnig A, Chen A, Lee YY, Adduci G, Choo A, Jungbauer A (2009) Adaptation of hybridomas to protein-free media results in a simplified two-step immunoglobulin M purification process. J Chromatogr A 1216:2683–2688
Liu CH, Chang TY (2006) Rational development of serum-free medium for Chinese hamster ovary cells. Process Biochem 41:2314–2319
Liu YF, Tang RH, Zhang QX, Shi JY, Li XM, Liu ZQ, Zhao W (1986) Stimulation of cell growth of Tetrahymena pyriformis and Chlamydomonas reinhardtii by trace elements. Biol Trace Elem Res 9:89–99
Manna L, Febo TD, Armillotta G, Luciani M, Ciarelli A, Salini R, Ventura MD (2015) Production of monoclonal antibodies in serum-free media. Monoclon Antib Immunodiagn Immunother 34:278–288
McGillicuddy N, Floris P, Albrecht S, Bones J (2018) Examining the sources of variability in cell culture media used for biopharmaceutical production. Biotechnol Lett 40:5–21
Nagira K, Hara T, Hayashida M, Osada K, Shiga M, Sasamoto K, Kina K, Murakami H (1995) Development of a protein-free medium with iron salts replacing transferrin for a human–human hybridoma. Biosci Biotechnol Biochem 59:743–745
Price PJ (2017) Best practices for media selection for mammalian cells. Vitro Cell Dev Biol Anim 53:673–681
Reimer CB, Phillips DJ, Aloisio CH, Moore DD, Galland GG, Wells TW, Black CM, McDougal JS (1984) Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma 3:263–275
Sen S, Roychoudhury PK (2013) Development of optimal medium for production of commercially important monoclonal antibody 520C9 by hybridoma cell. Cytotechnology 65:233–252
Shukla AA, Thömmes J (2010) Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol 28:253–261
Souza AS, Santos WNLD, Ferreira SLC (2005) Application of Box–Behnken design in the optimisation of an on-line pre-concentration system using knotted reactor for cadmium determination by flame atomic absorption spectrometry. Spectrochim Acta Part B 60:737–742
Spens E, Häggström L (2005) Defined protein-free NS0 myeloma cell cultures: stimulation of proliferation by conditioned medium factors. Biotechnol Prog 21:87–95
Tan KY, Teo KL, Lim JFY, Chen AKL, Reuveny S, Oh SKW (2015) Serum-free media formulations are cell line-specific and require optimization for microcarrier culture. Cytotherapy 17:1152–1165
van der Valk J, Brunner D, De Smet K, Fex Svenningsen Å, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G (2010) Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro 24:1053–1063
Xing Z, Kenty B, Koyrakh I, Borys M, Pan SH, Li ZJ (2011) Optimizing amino acid composition of CHO cell culture media for a fusion protein production. Process Biochem 46:1423–1429
Yao T, Asayama Y (2017) Animal-cell culture media: history, characteristics, and current issues. Reprod Med Biol 16:99–117
Yolmeh M, Jafari SM (2017) Applications of response surface methodology in the food industry processes. Food Bioprocess Technol 10:413–433
Zhang Y, Zhou Y, Yu J (1994) Effects of peptone on hybridoma growth and monoclonal antibody formation. Cytotechnology 16:147–150
Acknowledgements
We express gratitude to Ministry of Science and Technology (MOST 106-2221-E-182-050), Chang Gung University (BMRP 758) and Chang Gung Memorial Hospital (CMRPD2G0282, 2H0071) for funding and supporting this research. We would also like to thank the valuable suggestion of bioreactor operation from Frank R. H. Wang, United BioPharma Inc., Hsinchu, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors would like to declare that no conflicting financial interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, CH., Liu, YX. & Wu, WC. Facile development of medium optimization for antibody production: implementation in spinner flask and hollow fiber reactor. Cytotechnology 70, 1631–1642 (2018). https://doi.org/10.1007/s10616-018-0255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-018-0255-z