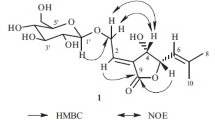
A new flavolignan acylglycoside, namely phillygenin 4-O-(2′′-trans-cinnamoyl)-α-L-rhamnopyranoside (1), along with a known phenolic acid (ellagic acid, 2) and three known flavonol glycosides (kaempferol 3-O-β-D-glucopyranoside (3), kaempferol 3-O-α-L-rhamnopyranoside (4), and quercetin 3-O-β-D-glucopyranoside (5), was isolated and purified from the fallen needles of Pinus banksiana by repeated column chromatography. The structures of these secondary metabolites were mainly elucidated by the extensive use of 1D and 2D NMR experiments, together with IR, UV, and FAB MS spectra. Among the four known compounds, 2 and 4 have never been found in this conifer species previously.

Similar content being viewed by others
References
L. K. Fu, N. Li, and R. M. Robert, Flora of China, 4, 11 (1999).
C. L. Si, J. Z. Jiang, S. C. Liu, H. Y. Hu, X. D. Ren, G. J. Yu, and G. H. Xu, Holzforschung, 67, 357 (2013).
J. Z. Wang, X. Y. Wang, and G. D. Zhao, J. Northeast Forestry Univ., 25, 72 (1997).
M. L. Liu, J. Liu, J. N. Li, and W. Zhao, J. Anhui Agri. Sci., 39, 15420 (2011).
M. Phelan, S. A. Aherne, A. Wong, and N. M. O′Brien, J. Med. Food, 12, 1245 (2009).
A. Pichette, F. X. Garneau, F. I. Jean, and B. Riedl, J. Wood Chem. Technol., 18, 427 (1998).
C. W. Beninger and M. M. Abou-Zaid, Biochem. Syst. Ecol., 25, 505 (1997).
C. Nozzolillo, P. Isabelle, O. M. Andersen, and M. Abou-Zaid, Can. J. Bot., 80, 796 (2002).
C. L. Si, G. H. Xu, X. F. Huang, Z. G. Du, L. Wu, and W. C. Hu, Chem. Nat. Compd., 52, 132 (2016).
C. L. Si, X. D. Ren, Z. G. Du, X. F. Huang, and L. Wu, Chem. Nat. Compd., 51, 1059 (2015).
J. Harmatha and L. Dinan, Phytochem. Rev., 2, 321 (2003).
H. Matsushita and T. Miyase-Uneo, Phytochemistry, 30, 2025 (1991).
V. V. Velde, D. Lavie, H. E. Gottlieb, G. W. Perold, and F. Scheinmann, J. Chem. Soc. Perkin Trans. 1, 1159 (1984).
T. Yahagi, A. Daikonya, and S. Kitanaka, Chem. Pharm. Bull., 60, 129 (2012).
M. A. R. Maiada, M. D. Paul, E. J. David, and A. L. John, Phytochemistry, 29, 1971 (1990).
D. K. Kim, J. P. Lim, J. W. Kim, H. W. Park, and J. S. Eun, Arch. Pharm. Res., 28, 39 (2005).
C. H. Gao, X. X. Yi, W. P. Xie, Y. N. Chen, M. B. Xu, Z. W. Su, L. Yu, and R. M. Huang, Mar. Drugs, 12, 4353 (2014).
C. L. Si, L. L. An, D. N. Xie, C. Y. Liu, X. Q. Chen, G. H. Wang, D. Huo, Q. L. Yang, and Y. M. Hong, Wood Sci. Technol., 50, 645 (2016).
D. Zhang, A. J. Deng, L. Ma, X. F. You, Z. H. Zhang, Z. H. Li, J. D. Jiang, and H. L. Qin, Phytochem. Lett., 12, 320 (2016).
Z. G. Tai, F. M. Zhang, L. Cai, J. Shi, Q. E. Cao, and Z. T. Ding, Chem. Nat. Compd., 48, 221 (2012).
D. D. Khac, S. Tran-Van, A. M. Campos, J. Y. Lallemand, and M. Fetizon, Phytochemistry, 29, 251 (1990).
D. J. Kwon and Y. S. Bae, Chem. Nat. Compd., 47, 636 (2011).
Acknowledgment
The authors would like to acknowledge Jiangsu Province Biomass Energy and Materials Laboratory in the Institute of the Chemical Industry of Forest Products, CAF (JSBEM201601), and Foundation (No. 201531) of Jiangsu Provincial Key Laboratory of Pulp and Paper Science and Technology, Nanjing Forestry University, P. R. China, Introduction of Overseas and Technical and Managerial Personnel Program (20173600003) of State Administration of Foreign Experts Affairs, P. R. China, for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2017, pp. 870–873.
Rights and permissions
About this article
Cite this article
Liu, CY., Xie, DN., An, LL. et al. Structural Elucidation of a New Flavolignan Acylglycoside from Fallen Needles of Pinus banksiana . Chem Nat Compd 53, 1020–1024 (2017). https://doi.org/10.1007/s10600-017-2192-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2192-z