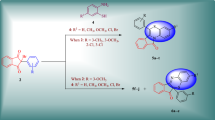
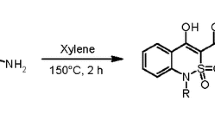
4,6-Dihydropyrano[3,2-c][2,1]benzothiazine 5,5-dioxides were synthesized via three-component interaction of 1-ethyl-1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide with active methylene nitriles and cycloalkanecarbaldehydes. The latter were also applied to obtain ammonium salts of 3,3'-(cycloalkylmethanediyl)bis(1-ethyl-1H-2,1-benzothiazin-4-ol) 2,2,2',2'-tetraoxides. The structures of the synthesized compounds were confirmed by 1H, 13C NMR as well as mass spectral and elemental analysis data. The structures of two representatives from each class of the target compounds have been additionally confirmed by single crystal X-ray diffraction study. The synthesized compounds were screened for antibacterial and antifungal activities.





Similar content being viewed by others
References
Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G.; Ed.; Elsevier, 2015.
Nadaraj, V.; Selvi, S. T.; Bai, H. P.; Mohan, S.; Thangadurai, T. D. Med. Chem. Res. 2012, 21, 2902.
Konkoy, C. S.; Fick, D. B.; Cai, S. X.; Lan, N. C.; Keana, J. F. W. US Patent 6680332B1.
Mountford, S. J.; Albiston, A. L.; Charman, W. N.; Ng, L.; Holien, J. K.; Parker, M. W.; Nicolazzo, J. A.; Thompson, P. E.; Chai, S. Y. J. Med. Chem. 2014, 57, 1368.
El-Bayouki, K. A. M.; Basyouni, W. M.; Khatab, T. K.; El-Basyoni, F. A.; Hamed, A. R.; Mostafa, E. A. J. Heterocycl. Chem. 2014, 51, 106.
Al-Masoudi, N. A.; Mohammed, H. H.; Hamdy, A. M.; Akrawi, O. A.; Eleya, N.; Spannenberg, A.; Pannecouque, C.; Langer, P. Z. Naturforsch., B: J. Chem. Sci. 2013, 68, 229.
El-Agrody, A. M.; Khattab, E. S. A. E. H.; Fouda, A. M.; Al-Ghamdi, A. M. Med. Chem. Res. 2012, 21, 4200.
Suárez, M.; Salfrán, E.; Verdecia, Y.; Ochoa, E.; Alba, L.; Martı́n, N.; Martínez, R.; Quinteiro, M.; Seoane, C.; Novoa, H.; Blaton, N.; Peeters, O. M.; De Ranter, C. Tetrahedron 2002, 58, 953.
(a) Asghari, S.; Ramezani, S.; Mohseni, M. Chin. Chem. Lett. 2014, 25, 431. (b) Kumar, D.; Reddy, V. B.; Sharad, S.; Dube, U.; Kapur, S. Eur. J. Med. Chem. 2009, 44, 3805. (c) Hossein nia, R.; Mamaghani, M.; Tabatabaeian, K.; Shirini, F.; Rassa, M. Bioorg. Med. Chem. Lett. 2012, 22, 5956. (d) Gunatilleke, S. S.; Calvet, C. M.; Johnston, J. B.; Chen, C.-K.; Erenburg, G.; Gut, J.; Engel, J. C.; Ang, K. K. H.; Mulvaney, J.; Chen, S.; Arkin, M. R.; McKerrow, J. H.; Podust, L. M. PLoS Neglected Trop. Dis. 2012, 6, e1736.
Litvinov, Y. M.; Shestopalov, A. M. In Advances in Heterocyclic Chemistry; Katritzky, A. R., Ed.; Elsevier: New York, 2011, Vol. 103, p. 175.
Heber, D.; Stoyanov, E. V. Synthesis 2003, 227.
Mosaddegh E.; Hassankhani, A. Catal. Commun. 2013, 33, 70.
Wu, Q.; Feng, H.; Guo, D.-D.; Yi, M.-S.; Wang, X.-H.; Jiang, B.; Tu, S.-J. J. Heterocycl. Chem. 2013, 50, 599.
Chen, T.; Xu, X.-P.; Ji, S.-J. J. Heterocycl. Chem. 2013, 50, 244.
Rajguru, D.; Keshwal, B. S.; Jain, S. Med. Chem. Res. 2013, 22, 5934.
Albadi, J.; Mansournezhad, A.; Darvishi-Paduk, M. Chin. Chem. Lett. 2013, 24, 208.
Azath, I. A.; Puthiaraj, P.; Pitchumani, K. ACS Sustainable Chem. Eng. 2013, 1, 174.
Azizi, K.; Heydari, A. RSC Adv. 2014, 4, 6508.
Baharfar, R.; Azimi, R. Synth. Commun. 2014, 44, 89.
Bazgir, A.; Hosseini, G.; Ghahremanzadeh, R. ACS Comb. Sci. 2013, 15, 530.
Davarpanah, J.; Kiasat, A. R.; Noorizadeh, S.; Ghahremani, M. J. Mol. Catal. A: Chem. 2013, 376, 78.
Ghalem, W.; Sedrati, R. H.; Benloucif, N.; Berree, F.; Boumoud, B.; Debache, A. Lett. Org. Chem. 2013, 10, 150.
Malakooti, R.; Parsaee, Z.; Hosseinabadi, R.; Oskooie, H. A.; Heravi, M. M.; Saeedi, M.; Amrollah, M.; Fallah, A. C. R. Chim. 2013, 16, 799.
Saha, M.; Pal, A. K. Synth. Commun. 2013, 43, 1708.
Shemchuk, L. A.; Lega, D. A.; Redkin, R. G.; Chernykh, V. P.; Shishkin, O. V.; Shishkina, S. V. Tetrahedron 2014, 70, 8348.
Lega, D. A.; Gorobets, N. Y.; Chernykh, V. P.; Shishkina, S. V.; Shemchuk, L. A. RSC Adv. 2016, 6, 16087.
Lega, D. A.; Chernykh, V. P.; Shemchuk, L. A. J. Org. Pharm. Chem. 2016, 14(1), 6.
Nie, H.; Widdowson, K. L. WO Patent 9834929, 1998; Chem. Abstr. 1998, 129, 1756451.
Iwatani, M.; Iwata, H.; Okabe, A.; Skene, R. J., Tomita, N., Hayashi, Y., Aramaki, Y.; David J. Hosfield Akira Hori, A.; Baba, A.; Miki, H. Eur. J. Med. Chem. 2013, 61, 49.
Yoakim, C.; O'Meara, J.; Simoneau, B.; Ogilie, W. W.; Deziel, R. WO Patent 2004026875, 2004; Chem. Abstr. 2004, 140, 303707.
Ukrainets, I. V.; Petrushova, L. A.; Dzyubenko, S. P.; Liu, Y. Chem. Heterocycl. Compd. 2014, 50, 564. [Khim. Geterotsikl. Soedin. 2014, 614.]
Fairhurst, J.; Gallagher, P. WO Patent 2001087881; Chem. Abstr. 2001, 136, 6015
Pieroni, M.; Sabatini, S.; Massari, S.; Kaatz, G. W.; Cecchetti, V.; Tabarrini, O. Med. Chem. Comm. 2012, 3, 1092.
Shafiq, M.; Zia-Ur-Rehman, M.; Khan, I. U.; Arshad, M. N.; Khan, S. A. J. Chil. Chem. Soc. 2011, 56, 527.
Goncharenko, M. P.; Sharanin, Y. A. Russ. J. Org. Chem. 1993, 29, 1218.
Shestopalov, A. M.; Emelianova, Y. M.; Nesterov, V. N. Russ. Chem. Bull. 2003, 52, 1164.
(a) Hough, W. L.; Rogers, R. D. Bull. Chem. Soc. Jpn., 2007, 80, 2262. (b) Lewandowski, P.; Kukawka, R.; Pospieszny, H.; Smiglak, M. New J. Chem. 2014, 38, 1372. (c) Kozlov, N. G.; Gusak, K. N.; Kadutskii, A. P. Chem. Heterocycl. Compd. 2010, 46, 505. [Khim. Geterotsikl. Soedin. 2010, 643.]
NMR Spectroscopy: An Introduction [Russian translation]; Gunther, H.; Mir: Moscow, 1984, p. 101.
CrysAlis PRO, Version 1.171.38.41; Rigaku Oxford Diffraction: Yarnton, 2015.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Farrugia, L. J. J. Appl. Crystallogr. 2012, 45, 849.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, D65, 148.
Guidelines for Susceptibility Testing of Microorganisms to Antibacterial Agents In Clinical Microbiology and Antimicrobial Chemotherapy [in Russian] 2004, 6, 306.
Ansel, H. C.; Norred, W. P.; Roth, I. L. J. Pharm. Sci. 1969, 58, 836.
David, N. A. Annu. Rev. Pharmacol. 1972, 12, 353.
We would like to express our sincere gratitude to prof. Nataliya I. Filimonova and to Ms. Elena V. Kirshenbaum(Department of Microbiology, Virology and Immunology,National University of Pharmacy, Kharkiv) for theirassistance in antimicrobial studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(2), 219–229
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 674 kb)
Rights and permissions
About this article
Cite this article
Lega, D.A., Chernykh, V.P., Zaprutko, L. et al. Synthesis of 1-ethyl-1H-2,1-benzothiazine 2,2-dioxide derivatives using cycloalkanecarbaldehydes and evaluation of their antimicrobial activity. Chem Heterocycl Comp 53, 219–229 (2017). https://doi.org/10.1007/s10593-017-2043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2043-7