Abstract
The microwave-induced three-component one-pot synthesis of 2-amino-3-carbethoxy-4-phenylpyrano[3,2-c]quinolin-5(6H)-ones (4a–l) from 4-hydroxyquinolin-2(1H)-ones, aromatic aldehydes and ethyl cyanoacetate was described. The detailed synthesis, structure analysis, and antimicrobial screening for the title compounds were also reported. Pharmacological screen studies like anti-inflammatory and antibacterial activities of newly synthesized quinoline derivatives were evaluated against carrageenan-induced rat paw edema model and Gram-positive and Gram-negative bacteria, respectively.
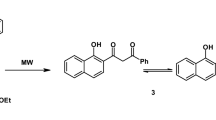
Graphical abstract
A base catalyzed three-component one-pot synthesis of pyrano[3,2-c]quinolines are reported. A selective angular cyclization product was obtained with good yield rather than linear product. Pharmacological screening of newly synthesized quinoline derivatives were evaluated against carrageenan induced rat paw edema model and bactericides.




Similar content being viewed by others
References
Armstrong RW, Combs AP, Tempest PA, Brown SD, Keating TA (1996) Multiple-component condensation strategies for combinatorial library synthesis. Acc Chem Res 29:123–131
Barr SA, Neville CF, Grundon MF, Boyd DR, Malone JF, Evans TA (1995) Quinolinone cycloaddition as a potential synthetic route to dimeric quinoline alkaloids. J Chem Soc Perkin Trans 1:445–452
Caddick S (1995) Microwave assisted organic reactions. Tetrahedron 51:10403–10432
Chen IS, Wu SJ, Tsai IL, Wu TS, Pezzuto JM, Lu MC, Chai H, Suh N, Teng CM (1994) Chemical and bioactive constituents from Zanthoxylum simulans. J Nat Prod 57:1206–1211
Collins CH, Lyne PM (1970) Microbial methods. Univesity Park Press, Baltimore
Daniel Thangadurai T, Jeong S, Yun S, Kim S, Kim C, Lee YI (2010) Antibacterial and luminescent properties of new donor–acceptor ruthenium triphenylphosphine–bipyridinium complexes. Microchem J 95:235–239
de Groot Ae, Jansen BJM (1975) A simple synthesis of 2 h-pyrans. A one-step synthesis of flindersine. Tetrahedron Lett 16:3407–3410
Huffman JW, Hsu TM (1972) A one step synthesis of flindersine. Tetrahedron Lett 13:141–143
Khurana JM, Kumar S (2009) Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett 50:4125–4127
Lee YR, Kweon HI, Koh WS, Min KR, Kim Y, Lee SH (2001) One-pot preparation of pyranoquinolinones by ytterbium(III) trifluoromethanesulfonate-catalyzed reactions: efficient synthesis of flindersine, N-methylflindersine, and zanthosimuline natural products. Synthesis 1851–1855
Li CJ (2005) Organic reactions in aqueous media with a focus on carbon–carbon bond formations: a decade update. Chem Rev 105:3095–3166
Majumdar KC, Mukhopadhyay PP (2003) Regioselective Synthesis of 2H-Benzopyrano-[3,2-c]quinolin-7(8H)-ones by radical cyclization. Synthesis 97–100
Manikandan S, Shanmugasundaram M, Raghunathan R (2002) Competition between two intramolecular domino Knoevenagel hetero Diels–Alder reactions: a new entry into novel pyranoquinolinone derivatives. Tetrahedron 58:8957–8962
Nadaraj V, Thamarai Selvi S (2007) Microwave-assisted solvent-free synthesis of 4-methyl-2-hydroxy- and 2-methyl-4-hydroxyquinoline. Indian J Chem 46B:1203–1207
Nadaraj V, Kalaivani S, Thamarai Selvi S (2006) An efficient synthesis of 9(10H)-acridinones under microwaves. Indian J Chem 45B:1958–1960
Nadaraj V, Thamarai Selvi S, Mohan S (2009) Microwave-induced synthesis and anti-microbial activities of 7,10,11,12-tetrahydrobenzo[c]acridin-8(9H)-one derivatives. Eur J Med Chem 44:976–980
Nadaraj V, Thamarai Selvi S, Daniel Thangadurai T (2011) Microwave synthesis of pyrimido[5,4-c]quinolones by modified Biginelli reaction and evaluation of their antimicrobial activity. J Pharm Res 4:1541–1544
Ramesh M, Mohan PS, Shanmugam P (1984) A convenient synthesis of flindersine, atanine and their analogues. Tetrahedron 40:4041–4049
Shwu Jen W, Ih Sheng C (1993) Alkaloids from Zanthoxylum simulans. Phytochemistry 34:1659–1661
Thamarai Selvi S, Nadaraj V, Mohan S, Sasi R, Hema M (2006) Solvent free microwave synthesis and evaluation of antimicrobial activity of pyrimido[4,5-b]- and pyrazolo[3,4-b]quinolones. Bioorg Med Chem 14:3896–3903
Tietze LF, Lieb ME (1998) Domino reactions for library synthesis of small molecules in combinatorial chemistry. Curr Opin Chem Biol 2:363–371
Ulubelen A, Meriçli AH, Meriçli F, Kaya Ü (1994) An alkaloid and lignans from Haplophyllum telephioides. Phytochemistry 35:1600–1601
Varma RS (1999) Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem 1:43–55
Winter CA, Risley EA, Nuss GW (1962) Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol NY 111:544–547
Ye JH, Ling KQ, Zhang Y, Li N, Yu JH (1999) Syntheses of 2-hydroxypyrano[3,2-c]quinolin-5-ones from 4-hydroxyquinolin-2-ones by tandem Knoevenagel condensation with aldehyde and Michael addition of enamine with the quinone methide—thermo- and photochemical approaches. J Chem Soc Perkin Trans 1:2017–2024
Acknowledgments
The financial support provided by the Government of Tamilnadu, India (to the author V. N.) is gratefully acknowledged. The authors thank Professor Yong-Ill Lee, Changwon National University, Changwon, Republic of Korea, and the Indian Institute of Science, Bangalore, India, for the spectral analyses.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nadaraj, V., Thamarai Selvi, S., Pricilla Bai, H. et al. Microwave solvent-free condition synthesis and pharmacological evaluation of pyrano[3,2-c]quinolines. Med Chem Res 21, 2902–2910 (2012). https://doi.org/10.1007/s00044-011-9810-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9810-2