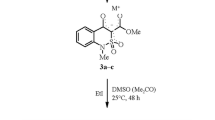
The reaction of alkyl 1-R-4-hydroxy-2,2-dioxo-1Н-2λ6,1-benzothiazine-3-carboxylates with 1H-1,2,4-triazol-5-amine in refluxing xylene produced not only 1-R-4-hydroxy-2,2-dioxo-N-(1H-1,2,4-triazol-5-yl)-1H-2λ6,1-benzothiazine-3-carboxamides, but also 6,6-dioxides of 7-R-3H-[1,2,4]triazolo[5',1':2,3]pyrimido[5,4-c][2,1]benzothiazin-5(7H)-ones.


Similar content being viewed by others
References
Ukrainets, I. V.; Petrushova, L. A.; Shishkina, S. V.; Sim, G. Chem. Heterocycl. Compd. 2014, 50, 1741. [Khim. Geterotsikl. Soedin. 2014, 1892.]
(a) Abdel-Megeed, A. M.; Abdel-Rahman, H. M.; Alkaramany, G. E.; El-Gendy, M. A. Eur. J. Med. Chem. 2009, 44, 117. (b) Furukawa, A.; Oikawa, S.; Harada, K.; Sugiyama, H.; Hiraku, Y.; Murata, M.; Shimada, A.; Kawanishi, S. Mutat. Res. 2010, 694, 7. (c) Chilumuri, A.; Odell, M.; Milton, N. G. N. ACS Chem. Neurosci. 2013, 4, 1501. (d) Paul, K. B.; Hedge, J. M.; Rotroff, D. M.; Hornung, M. W.; Crofton, K. M.; Simmons, S. O. Chem. Res. Toxicol. 2014, 27, 387. (e) Valenti, V. E.; de Abreu, L. C.; Fonseca, F. L. A.; Figueiredo, J.-L.; Adami, F.; Ferreira, C. Int. J. Health. Sci. (Qassim) 2013, 7, 200. (f) Jeong, J. S.; Suh, J. K.; Cho, E. S.; Kim, D. W.; Jeong, M. A. Korean J. Anesthesiol. 2013, 65, 552.
(a) Huang, L. H.; Zheng, Y. F.; Song, C. J.; Wang, Y. G.; Xie, Z. Y.; Lai, Y. W.; Lu, Y. Z.; Liu, H. M. Steroids 2012, 77, 367. (b) Huang, L. H.; Zheng, Y. F.; Lu, Y. Z.; Song, C. J.; Wang, Y. G.; Yu, B.; Liu, H. M. Steroids 2012, 77, 710.
(a) Ablajan, K.; Kamil, W.; Tuoheti, A.; Wan-Fu. S. Molecules 2012, 17, 1860. (b) Kolosov, M. A.; Kulik, O. G.; Chepeleva, L. V.; Orlov, V. D. Visn. Kharkiv. Nats. Un-tu 2013, No. 1085. Khimiya, Vip. 22(45), 39. (с) Lipson, V. V.; Desenko, S. M.; Orlov, V. D.; Shishkin, O. V.; Shirobokova, M. G.; Chernenko, V. N.; Zinov'eva, L. I. Chem. Heterocycl. Compd. 2000, 36, 1329. [Khim. Geterotsikl. Soedin. 2000, 1542.]
(a) Kril'skiy, D. V.; Shikhaliev, Kh. S.; Falaleev, A. V.; Kovygin, Yu. A. Vestn. Voronezh. Gos. Un-ta. Ser.: Khimiya. Biologiya. Farmatsiya 2005, (1), 58. (b) Lipson, V. V.; Orlov, V. D.; Desenko, S. M.; Karnozhitskaya, T. M.; Shirobokova, M. G. Chem. Heterocycl. Compd. 1999, 35, 595. [Khim. Geterotsikl. Soedin. 1999, 664.] (c) Voronkov, M. G.; Shagun, L. G.; Dorofeev, I. A.; Zhilitskaya, L. V.; Yarosh, N. O.; Larina, L. I. Russ. Chem. Bull., Int. Ed. 2013, 62, 2554. [Izv. AN, Ser. Khim. 2013, 2554.]
Fidler, Zh. N.; Shibanova, E. F.; Makerov, P. V.; Kalikhman, I. D.; Shulunova, A. M.; Sarapulova, G. I.; Klyba, L. V.; Vitkovskii, V. Yu.; Chipanina, N. N.; Lopyrev, V. A.; Voronkov, M. G. Chem. Heterocycl. Compd. 1980, 16, 1079. [Khim. Geterotsikl. Soedin. 1980, 1414.]
Boboshko, L. G.; Zubritskii, M. Yu.; Kovalenko, V. V.; Popov, A. F.; Rybakov, V. B.; Savelova, V. A.; Mikhailov, V. A. Zh. Org. ta Farm. Khim. 2011, 9 (4), 42.
(a) Barmin, M. I.; Mel'nikov, А. А.; Kartavykh, V. P.; Mel'nikov, V. V. Izv. vuzov. Khimiya i Khim. Tekhnol. 2005, 48 (9), 103. (b) Sorescu, D. C.; Bennett, C. M.; Thompson, D. L. J. Phys. Chem. 1998, 102A, 10348. (c) Fuentes, J. J.; Lenoir, J. A. Can. J. Chem. 1976, 54, 3620. (d) Chang, H.; Kim, K.; Ree, M.; Lee, K.-W. Macromol. Chem. Phys. 1999, 200, 422.
(a) Elguero, J.; Katritzky, A. R.; Denisko, O. V. In Advances in Heterocyclic Chemistry, Katritzky, A. R., Ed.; Elsevier: New York, 2000, vol. 76, p. 1. (b) Minkin, V. I.; Garnovskii, A. D.; Elguero, J.; Katritzky, A. R.; Denisko, O. V. In Advances in Heterocyclic Chemistry, Katritzky, A. R., Ed.; Elsevier: New York, 2000, vol. 76, p. 157.
Ukrainets, I. V.; Petrushova, L. A.; Dzyubenko, S. P. Zh. Org. ta Farm. Khim. 2014 , 12 (2), 53.
(a) Ukrainets, I. V.; Petrushova, L. A.; Dzyubenko, S. P.; Sim, G. Chem. Heterocycl. Compd. 2014, 50, 103. [Khim. Geterotsikl. Soedin. 2014, 114.] (b) Ukrainets, I. V.; Petrushova, L. A.; Dzyubenko, S. P.; Yangyang, L. Chem. Heterocycl. Compd. 2014, 50, 564. [Khim. Geterotsikl. Soedin. 2014, 614.]
Zefirov, N. S.; Palyulin, V. A.; Dashevskaya, E. E. J. Phys. Org. Chem. 1990, 3, 147.
Orpen, A. G.; Brammer, L.; Allen, F. H.; Kennard, O.; Watson, D. G.; Taylor, R. In Structure Сorrelation, Burgi, H.-B.; Dunitz, J. D., Eds.; VCH: Weinheim, 1994, vol. 2, p. 741.
Zefirov, Yu. V. Crystallogr. Rep. 1997, 42, 865. [Kristallografiya 1997, 42, 936.]
Ukrainets, I. V.; Sidorenko, L. V.; Gorokhova, O. V. Chem. Heterocycl. Compd. 2005, 41, 896. [Khim. Geterotsikl. Soedin. 2005, 1060.]
Günther, H. NMR Spectroscopy: Basic Principles, Concepts, and Applications in Chemistry, Wiley-VCH: Weinheim, 2013, p. 114.
Ukrainets, I. V.; Petrushova, L. A.; Dzyubenko, S. P. Chem. Heterocycl. Compd. 2013, 49, 1378. [Khim. Geterotsikl. Soedin. 2013, 1479.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(1), 97–101
* For Communication 9, see.1
Rights and permissions
About this article
Cite this article
Ukrainets, I.V., Petrushova, L.A., Sim, G. et al. 2,1-Benzothiazine 2,2-dioxides 10*. Reaction of alkyl 1-R-4-hydroxy-2,2-dioxo-1Н-2λ6,1-benzothiazine-3-carboxylates with 1H-1,2,4-triazol-5-amine. Chem Heterocycl Comp 51, 97–101 (2015). https://doi.org/10.1007/s10593-015-1665-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1665-x