Abstract
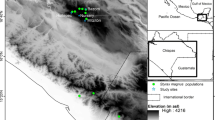
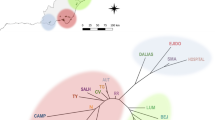
Evaluating the consequences of the decline of threatened species on their population genetic structure is crucial for establishing effective conservation strategies in the strongly fragmented landscapes of Central Europe. Laserpitium prutenicum is a bi- to perennial forb occurring in intermittently wet meadows and light oak forests throughout central to eastern and south-eastern Europe. During the past 70 years, the western limit of its distributional range retracted dramatically, the number of populations decreased and the remaining populations faced a considerable increase of fragmentation. To study the effects of this decline on the genetic diversity of L. prutenicum, we conducted an AFLP study on 20 populations from Germany, Poland and the Czech Republic. For comparison, we collected the same data on Selinum carvifolia, a taxonomically related and both ecologically and morphologically similar species, which is still more common in the study area. Both species showed similarly weak spatial genetic structuring and intermediate genetic diversities. We attribute this result to the loss of habitat being faster than the loss of genetic diversity in smaller and fragmented populations. Depending on the ecological characteristics of a species, even a gradual disappearance is not necessarily accompanied by any detectable effect at the population genetic level (“silent goodbye”). In the case of L. prutenicum, habitat preservation should be given priority over all other conservation measures.




Similar content being viewed by others
References
Ægisdóttir HH, Kuss P, Stöcklin J (2009) Isolated populations of a rare alpine plant show high genetic diversity and considerable population differentiation. Ann Bot 104:1313–1322. doi:10.1093/aob/mcp242
Barringer BC, Kulka EA, Galloway LF (2012) Reduced inbreeding depression in peripheral relative to central populations of a monocarpic herb. J Evol Biol 25:1200–1208. doi:10.1111/j.1420-9101.2012.02510.x
Bennett AF, Saunders DA (2010) Habitat fragmentation and landscape change. In: Sodhi NS, Ehrlich PR (eds) Conservation biology for all. Oxford University, Oxford, pp 1544–1550
Böhnert W, Gutte P, Schmidt PA (2001) Verzeichnis und Rote Liste der Pflanzengesellschaften Sachsens. Sächsisches Landesamt für Umwelt und Geologie, Dresden
Brütting C, Meyer S, Kühne P et al (2012a) Spatial genetic structure and low diversity of the rare arable plant Bupleurum rotundifolium L. indicate fragmentation in Central Europe. Agric Ecosyst Environ 161:70–77. doi:10.1016/j.agee.2012.07.017
Brütting C, Wesche K, Meyer S, Hensen I (2012b) Genetic diversity of six arable plants in relation to their red list status. Biodivers Conserv 21:745–761. doi:10.1007/s10531-011-0212-z
Bylebyl K, Poschlod P, Reisch C (2008) Genetic variation of Eryngium campestre L. (Apiaceae) in Central Europe. Mol Ecol 17:3379–3388. doi:10.1111/j.1365-294X.2008.03836.x
Cavagnaro PF, Chung S-M, Manin S et al (2011) Microsatellite isolation and marker development in carrot—genomic distribution, linkage mapping, genetic diversity analysis and marker transferability across Apiaceae. BMC Genom 12:386. doi:10.1186/1471-2164-12-386
Chytrý M (ed) (2013) Vegetace České republiky 4. Lesní a křovinná vegetace/Vegetation of the Czech Republic 4. Forest and Scrub Vegetation, Vyd. 1. Academia, Praha
Cole CT (2003) Genetic variation in rare and common plants. Annu Rev Ecol Evol Syst 34:213–237. doi:10.1146/annurev.ecolsys.34.030102.151717
Di Giulio M, Holderegger R, Tobias S (2009) Effects of habitat and landscape fragmentation on humans and biodiversity in densely populated landscapes. J Environ Manag 90:2959–2968. doi:10.1016/j.jenvman.2009.05.002
Dittbrenner A, Hensen I, Wesche K (2005) Genetic structure and random amplified polymorphic DNA diversity of the rapidly declining Angelica palustris (Apiaceae) in Eastern Germany in relation to population size and seed production. Plant Species Biol 20:191–200
Dreier S, Redhead JW, Warren IA et al (2014) Fine-scale spatial genetic structure of common and declining bumble bees across an agricultural landscape. Mol Ecol 23:3384–3395. doi:10.1111/mec.12823
Dumolin S, Demesure B, Petit RJ (1995) Inheritance of chloroplast and mitochondrial genomes in pedunculate oak investigated with an efficient PCR method. Theor Appl Genet 91:1253–1256. doi:10.1007/BF00220937
Durka W (2002) Blüten- und Reproduktionsbiologie. In: Klotz S, Kühn I, Durka W (eds) BIOLFLOR—eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. Bundesamt für Naturschutz, Bonn, pp 133–175
Durka W, Nossol C, Welk E et al (2013) Extreme genetic depauperation and differentiation of both populations and species in Eurasian feather grasses (Stipa). Plant Syst Evol 299:259–269. doi:10.1007/s00606-012-0719-0
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol Ecol 17:1170–1188. doi:10.1111/j.1365-294X.2007.03659.x
Eichel L (2013) Genetik und Gefährdung zweier unterschiedlich häufiger Apiaceenarten am Beispiel Selinum carvifolia und Laserpitium prutenicum. Diplom thesis, Martin-Luther-Universität Halle-Wittenberg
Ellenberg H, Düll R, Wirth V et al (1992) Zeigerwerte von Pflanzen in Mitteleuropa, 2nd edn. Scripta Geobotanica 18, Verlag Erich Goltze KG, Göttingen
Gaudeul M, Till-Bottraud I, Barjon F, Manel S (2004) Genetic diversity and differentiation in Eryngium alpinum L. (Apiaceae): comparison of AFLP and microsatellite markers. Heredity 92:508–518. doi:10.1038/sj.hdy.6800443
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Grulich V (2012) Red List of vascular plants of the Czech Republic: 3rd edition—Červený seznam cévnatých rostlin České republiky: třetí vydání. Preslia 84:631–645
Guo Q, Taper M, Schoenberger M, Brandle J (2005) Spatial-temporal population dynamics across species range: from centre to margin. Oikos 108:47–57
Hardtke H-J, Ihl A (2000) Atlas der Farn- und Samenpflanzen Sachsens. Sächsisches Landesamt für Umwelt und Geologie, Dresden
Hegi G (1975) Umbelliferae (Doldengewächse). In: Hegi G (ed) Illustrierte Flora von Mitteleuropa, Band V, Teil 2, Dicotyledones Teil 3.2: Cactaceae-Cornaceae, 2nd edn. Paul Parey Verlag, Berlin
Hempel W (1972) Waldsteppenpflanzen der Oberlausitz. Abh Ber des Naturkundemuseums Görlitz 47:1–16
Hempel W (2009) Die Pflanzenwelt Sachsens von der Späteiszeit bis zur Gegenwart, 1st edn. Weissdorn-Verlag, Jena
Hensen I, Oberprieler C, Wesche K (2005) Genetic structure, population size, and seed production of Pulsatilla vulgaris Mill. (Ranunculaceae) in Central Germany. Flora 200:3–14. doi:10.1016/j.flora.2004.05.001
Hogbin PM, Peakall R (1999) Evaluation of the contribution of genetic research to the management of the endangered plant Zieria prostrata. Conserv Biol 13:514–522. doi:10.1046/j.1523-1739.1999.98182.x
Honnay O, Jacquemyn H (2007) Susceptibility of common and rare plant species to the genetic consequences of habitat fragmentation. Conserv Biol 21:823–831. doi:10.1111/j.1523-1739.2006.00646.x
Jäger EJ (ed) (2011) Rothmaler—Exkursionsflora von Deutschland. Gefäßpflanzen: Grundband, 20th edn. Spektrum Akademischer Verlag, Heidelberg
Johnson WE, Onorato DP, Roelke ME et al (2010) Genetic restoration of the Florida panther. Science 329:1641–1645. doi:10.1126/science.1192465
Kącki Z, Michalska-Hejduk D (2010) Assessment of biodiversity in Molinia meadows in Kampinoski National Park based on biocenotic indicators. Pol J Environ Stud 19:351–362
Kącki Z, Dajdok Z, Szcęśniak E (2003) Czerwona lista roślin naczyniowych Dolnego Śląska. In: Kącki Z (ed) Zagrożone gatunki flory naczyniowej Dolnego Śląska. Instytut Biologii Roślin UWr, Polskie Towarzystwo Przyjaciół Przyrody “pro Natura”, Wrocław, pp 19–56
Kącki Z, Dajdok Z, Szczęśniak E (2009) Proposed standardized criteria for regional evaluation of the level of threat to plant species, based on studies in Lower Silesia, Poland. In: Mirek Z, Nikel A (eds) Rare, relict and endangered plants and fungi in Poland”. W. Szafer Institute of Botany. Polish Academy of Sciences, Kraków, pp 19–30
Kącki Z, Czarniecka M, Swacha G (2013) Statistical determination of diagnostic, constant and dominant species of the higher vegetation units in Poland. Polish Botanical Society, Łódź
Konold W, Burkart B (eds) (2003) Offenland & Naturschutz. Verlag des Instituts für Landespflege der Universität Freiburg, Freiburg im Breisgau
Kress JW, Maddox D, Roesel CS (1994) Genetic variation and protection priorities in Ptilimnium nodosum (Apiaceae), an endangered plant of the eastern United States. Conserv Biol 8:271–276
Kühn I, Durka W, Klotz S (2004) BiolFlor—a new plant-trait database as a tool for plant invasion ecology. Divers Distrib 10:363–365. doi:10.1111/j.1366-9516.2004.00106.x
Lammi A, Siikamäki P, Mustajärvi K (1999) Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conserv Biol 13:1069–1078. doi:10.1046/j.1523-1739.1999.98278.x
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Lauterbach D, Ristow M, Gemeinholzer B (2011) Genetic population structure, fitness variation and the importance of population history in remnant populations of the endangered plant Silene chlorantha (Willd.) Ehrh. (Caryophyllaceae). Plant Biol 13:667–777. doi:10.1111/j.1438-8677.2010.00418.x
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952. doi:10.1111/j.1365-2745.2006.01150.x
Linhart YB, Premoli AC (1993) Genetic variation in Aletes acaulis and its relative, the narrow endemic A. humilis (Apiaceae). Am J Bot 80:598–605. doi:10.2307/2445378
Londo G (1976) The decimal scale for releves of permanent quadrats. Vegetatio 33:61–64. doi:10.1007/BF00055300
López-Pujol J, Bosch M, Simon J, Blanche C (2002) Allozyme variation and population structure of the very narrow endemic Seseli farrenyi (Apiaceae). Bot J Linn Soc 138:305–314
Ludwig G, Schnittler M (1996) Rote Liste gefährdeter Pflanzen Deutschlands. Bundesamt für Naturschutz, Bonn
Lundqvist J, Jäger EJ (eds) (1995) Dicotyledonae K-M. Swedish Museum of Natural History, Stockholm
Marchi A, Appendino G, Pirisi I et al (2003) Genetic differentiation of two distinct chemotypes of Ferula communis (Apiaceae) in Sardinia (Italy). Biochem Syst Ecol 31:1397–1408. doi:10.1016/S0305-1978(03)00117-0
Metcalf JC, Rose KE, Rees M (2003) Evolutionary demography of monocarpic perennials. Trends Ecol Evol 18:471–480. doi:10.1016/S0169-5347(03)00162-9
Meusel H, Jäger EJ, Rauschert S, Weinert E (1978) Vergleichende Chorologie der zentraleuropäischen Flora. VEB Gustav Fischer Verlag, Jena
Mieder N (2012) Mikrosatellitenanalyse in ostsächsischen Laserpitium prutenicum (Apiaceae) Populationen. Research Internship Report, Hochschule Zittau/Görlitz
Moritz C (2002) Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst Biol 51:238–254
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155. doi:10.1111/j.1365-294X.2004.02141.x
Nybom H, Bartish IV (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst 3:93–114
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. doi:10.1111/j.1471-8286.2005.01155.x
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539. doi:10.1093/bioinformatics/bts460
Pfeifer M, Schatz B, Xavier Picó F et al (2009) Phylogeography and genetic structure of the orchid Himantoglossum hircinum (L.) Spreng. across its European central–marginal gradient. J Biogeogr 36:2353–2365. doi:10.1111/j.1365-2699.2009.02168.x
Poschlod P (2015) Geschichte der Kulturlandschaft: Entstehungsursachen und Steuerungsfaktoren der Entwicklung der Kulturlandschaft, Lebensraum- und Artenvielfalt in Mitteleuropa. Verlag Eugen Ulmer, Stuttgart
Procházková J (2010) Obsah DNA a AT/GC genomový poměr v čeledi Apiaceae. Diplom thesis, Masaryk University
Qiu Y-X, Hong D-Y, Fu C-X, Cameron KM (2004) Genetic variation in the endangered and endemic species Changium smyrnioides (Apiaceae). Biochem Syst Ecol 32:583–596. doi:10.1016/j.bse.2003.08.004
R Development Core Team (2014) R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna
Ralls K, Ballou JD (2004) Genetic status and management of California condors. Condor 106:215–228. doi:10.1650/7348
Reichel K (2012) Populationsbiologische Untersuchungen an Laserpitium prutenicum (Apiaceae) in Ostsachsen und angrenzenden Gebieten. Diplom thesis, Technische Universität Dreden
Reichel K (2013) 70 Jahre nach Theodor Schütze—Das Preußische Laserkraut (Laserpitium prutenicum) in Ostsachsen und angrenzenden Gebieten. Ber der Naturforschenden Ges der Oberlausitz 21:17–30
Rennwald E (ed) (2000) Verzeichnis und Rote Liste der Pflanzengesellschaften Deutschlands: Referate und Ergebnisse des gleichnahmigen Fachsymposiums in Bonn von 30.06.−02.07.2000. Bundesamt für Naturschutz, Bonn
Richter F (2014) Umweltwandel in der sächsischen Lausitz am Beispiel von Arnica montana und Gladiolus imbricatus. Peckiana 9:105–117
Röder M, Syrbe R-U, Bastian O (1999) Bodenveränderungen und Landschaftswandel im Biosphärenreservat Oberlausitzer Heide- und Teichlandschaft. Erde 130:297–313
Sala OE, Chapin FS III et al (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. doi:10.1126/science.287.5459.1770
Schönswetter P, Tribsch A (2005) Vicariance and dispersal in the alpine perennial Bupleurum stellatum L. (Apiaceae). Taxon 54:725. doi:10.2307/25065429
Schulz (2013) Rote Liste und Artenliste Sachsens: Farn- und Samenpflanzen. Sächsisches Landesamt für Umwelt, Landwitschaft und Geologie
Schütze T (1940) Das Preußische Laserkraut (Laserpitium prutenicum L.)—eine characteristische Hochsommerpflanze der Oberlausitz. Isis Budissina 14:34–44
Severns P (2003) Inbreeding and small population size reduce seed set in a threatened and fragmented plant species, Lupinus sulphureus ssp. kincaidii (Fabaceae). Biol Conserv 110:221–229. doi:10.1016/S0006-3207(02)00191-X
Tackenberg O (2001) Methoden zur Bewertung gradueller Unterschiede des Ausbreitungspotentials von Pflanzenarten: Modellierung des Windausbreitungspotentials und regelbasierte Ableitung des Fernausbreitungspotentials. Dissertation, Philipps-Universität Marburg
Tackenberg O, Poschlod P, Bonn S (2003) Assessment of wind dispersal potential in plant species. Ecol Monogr 73:191–205
Van Rossum F, De Sousa SC, Triest L (2004) Genetic consequences of habitat fragmentation in an agricultural landscape on the common Primula veris, and comparison with its rare congener, P. vulgaris. Conserv Genet 5:231–245
Vitalis R, Glémin S, Olivieri I (2004) When genes go to sleep: the population genetic consequences of seed dormancy and monocarpic perenniality. Am Nat 163:295–311. doi:10.1086/381041
Vos P, Hogers R, Bleeker M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wagner V, von Wehrden H, Wesche K et al (2011) Similar performance in central and range-edge populations of a Eurasian steppe grass under different climate and soil pH regimes. Ecography 34:498–506. doi:10.1111/j.1600-0587.2010.06658.x
Wagner V, Treiber J, Danihelka J et al (2012) Declining genetic diversity and increasing genetic isolation toward the range periphery of Stipa pennata, a Eurasian feather grass. Int J Plant Sci 173:802–811. doi:10.1086/666663
Weising K, Nybom H, Pfenninger M et al (2005) DNA fingerprinting in plants: principles, methods, and applications, second edition, 2nd edn. CRC Press Taylor & Francis Group, Boca Raton
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 10:413–418
Zarzycki K, Szeląg Z (2006) Red list of the vascular plants in Poland/Czerwona lista roślin naczyniowych w Polsce. In: Mirek Z, Zarzycki K, Wojewoda W, Szeląg Z (eds) Red list of plants and fungi in Poland/Czerwona lista roślin i grzybów Polski. W. Szafer Institute of Botany. Polish Academy of Sciences, Kraków
Zieverink M, Walczak C, Schmidt PA (2010) Regeneration und Verbund (sub-)montaner Grünlandbiotope im Osterzgebirge: Ergebnisse eines E + E-Vorhabens des Bundesamtes für Naturschutz. Bundesamt für Naturschutz, Bonn
Acknowledgments
We are indebted to A. Beck, W. Bena, V. Dittmann, P. Gutte, K. Kubat, M. Schrack, A. Schurig, J. Tischer, C. Walczak, A. Wünsche, B. Zöphel and the Sächsisches Landesamt für Umwelt, Landwirtschaft und Geologie for assistance in locating our study populations. The local conservation agencies (Untere Naturschutzbehörden Görlitz, Bautzen, Sächsische Schweiz-Osterzgebirge) and the biosphere reserve Oberlausitzer Heide- und Teichlandschaft kindly permitted our research on protected sites, while the Landesverein Sächsischer Heimatschutz and the BHW Basaltwerk Mittelherwigsdorf oHG allowed us to access populations on their properties. C. Walczak and M. Zieverink helped to plan the study, for which P. Gebauer, N. Mieder and M. Schwager provided excellent technical support. We are especially grateful to R. Reichel for adapting the Genographer user interface to our needs. The presentation benefitted from comments by two anonymous referees and the handling editor. Our study was partially financed by the German-Czech Erhaltungsprojekt für seltene Pflanzen im Offenland von Böhmen und Sachsen (EPOBS, project leader F. Müller; TU Dresden), a part of the European Union’s Ziel3/Cíl3 initiative.
Data accessibility
Our datasets (population level ecological data, AFLP fingerprints of all individuals) are available as online resource A7. The source code of the modified version of the Genographer software we used for scoring is available on request from the corresponding author under the GPL 2 license. It is based on Genographer version 2.1.4, with modifications only affecting the user interface (automatic rescoring disabled, increased zoom range, output table adapted for AFLP data).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10592_2016_827_MOESM1_ESM.png
Online resource A1 Distribution ranges for L. prutenicum ssp. prutenicum and S. carvifolia in Europe. Solid grey—main range and outpost populations (DOTS) of S. carvifolia; black dashed—main range of L. prutenicum ssp. prutenicum; other dots: outpost populations of L. prutenicum ssp. prutenicum observed between 2000 and 2011 (big black), 1980–2000 (small black), 1950–1980 (big white), 1800–1950 (small white) or at an unknown time (grey square). The map was compiled from published distribution maps (Meusel et al. 1978), occurrence data from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org), national and regional floristic databases, and further maps found in the floristic literature (bibliographic details given in Index Holmiensis: Lundqvist and Jäger 1995). During mapping, contiguous large areas of occurrence, where the species were reported to be evenly distributed, were generalized as range polygons; spatially isolated occurrences were digitized as single point locations. White rectangle marks study area (PNG 254 kb)
10592_2016_827_MOESM2_ESM.pdf
Online resource A2 Table of further genetic diversity indices calculated for L. prutenicum and S. carvifolia. Samples—total no. of sampled individuals, PPL—percent polymorphic loci; I—Shannon’s index of information; He—expected heterozygosity; uHe expected heterozygosity corrected for sample size. For both species, values are given for all samples and loci and for the mean of ten subsamples of ten samples per population (where possible). For S. carvifolia, values are also given for ten subsamples with 66 randomly selected loci to match the no. of loci used in L. prutenicum (PDF 29 kb)
10592_2016_827_MOESM3_ESM.tiff
Online resource A3 Scatter plot showing the correlation between the geographical and the genetic distance (PhiST) for L. prutenicum (a) and S. carvifolia (b). Mantel tests revealed no significant correlation between genetic and geographic distances in L. prutenicum (Rxy = −0.069; p = 0.377). In S. carvifolia geographical distances were negatively correlated with genetic distances (Rxy = −0.266; p = 0.013). The maximal geographical distance between S. carvifolia populations was smaller since this species was sampled in the core study area only (TIFF 148 kb)
10592_2016_827_MOESM4_ESM.pdf
Online resource A4 Spearman rank correlations between genetic diversity, presented as percentage of polymorphic loci (excluding populations with <10 sampled individuals), ecological and fitness data in L. prutenicum (a) and S. carvifolia (b). Correlation coefficients rS below the diagonal, p-values above the diagonal. Legend and overview of the data basis (missing values) on second page (PDF 66 kb)
10592_2016_827_MOESM5_ESM.tiff
Online resource A5 Scatter plot showing the correlation between the clay content and the weight of diaspores for L. prutenicum (black triangles; rS = −0.306; p = 0.231) and S. carvifolia (grey dots, rS = −0,767; p = 0.021) (TIFF 86 kb)
10592_2016_827_MOESM6_ESM.tiff
Online resource A6 Scatter plot showing the correlation between the magnesium content and the weight of diaspores for L. prutenicum (black triangles, rS = −0.056; p = 0.831) and S. carvifolia (grey dots, rS = −0.717; p = 0.037) (TIFF 94 kb)
10592_2016_827_MOESM7_ESM.xlsx
Online resource A7 Population genetic data and ecological data for the studied populations. AFLP fingerprints for 66 polymorphic fragments from 291 individuals in 20 populations of L. prutenicum and for 172 polymorphic fragments from 278 individuals in 16 populations of S. carvifolia. The ecological dataset contains species, region, latitude (N), longitude (E), elevation, no. of genetic samples, size class, standardized population size, PPL standardized for 10 individuals, number of plants sampled for diaspores, mean diaspore weight, soil Ca, soil K, soil Mg, soil P, total soil N, soil pH, soil electric conductivity, soil clay content, soil humus content, soil C/N ratio, Ellenberg Indicator Values for light, temperature, continentality, humidity, soil reaction and nutrients, and the cover ratio of Ellenberg Indicator species for intermittent water regime/flooding (XLSX 292 kb)
Rights and permissions
About this article
Cite this article
Reichel, K., Richter, F., Eichel, L. et al. Genetic diversity in the locally declining Laserpitium prutenicum L. and the more common Selinum carvifolia (L.) L.: a “silent goodbye”?. Conserv Genet 17, 847–860 (2016). https://doi.org/10.1007/s10592-016-0827-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0827-4