Abstract
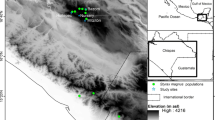
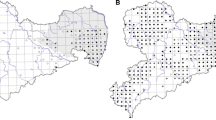
The success of restoration projects depends on the genetic diversity of the implanted species. It is a limiting factor, often because the seed sources are immersed in highly fragmented landscapes. In this work, we compare the genetic diversity of the juveniles, and the adult trees of Schinus terebinthifolia Raddi in a native mixed-species planting, both in the restoration process and in the remaining natural vegetation Atlantic Forest Biome. Polymorphic DNA fragments using five SSR primers were used to estimate the restored population that showed a higher genetic diversity index (He) (0.553 adults and 0.505 juveniles) compared to the wild population (0.487 adults and 0.483 juveniles). The forested area was established with individuals of high genetic diversity. There is a reduced genetic diversity for juveniles, with the loss of exclusive alleles and maintenance of endogamy and coancestry in reforested populations, and we can infer that there was a low gene flow inter fragments. The effective population size in both (adults and juveniles) was lower than the value recommended for the sustainability of populations in the short and long term. The results indicated that continuous monitoring of this particular area is of absolute necessity and should use techniques that promote the connectivity of the fragments. It would allow for a more significant reduction of genetic drift and the persistence of the planted populations.

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Álvares-Carvalho SV et al (2015) Schinus terebinthifolia: population structure and implications for its conservation. Biochem Syst Ecol 58:120–125
Álvares-Carvalho SV et al (2016) Structure and genetic diversity of natural Brazilian pepper populations (Schinus terebinthifolia Raddi). Genet Mol Res 15:1–13
Álvares-Carvalho SV et al (2017) Restoration over time and sustainability of Schinus terebinthifolia Raddi. Genet Mol Res 16:1–9
Cruz Neto O et al (2014) Genetic and ecological outcomes of Inga vera subsp. affinis (Leguminosae) tree plantations in a fragmented tropical landscape. PLoS ONE 9:1–8
de Siqueira LP, Tedesco AM, Rodrigues RR, Chaves RB, Albuquerque NC, Corrêa FF, Santiami EL, Tambosi LR, Guimarães TM, Brancalion PH (2021) Engaging people for large-scale forest restoration: governance lessons from the Atlantic Forest of Brazil. In: The Atlantic Forest. Springer, Cham, pp 389–402
Frankham R, Bradshaw CJA, Brook BW (2014) Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol Conserv 170:56–63
Grelle CEV, Niemeyer J, Castro EBV, Lanna AM, Uzeda M, Vieira MV (2021) Conservation initiatives in the Brazilian Atlantic forest. In: The Atlantic Forest. Springer, Cham, pp. 421–449
González AV, Gómez-Silva V, Ramírez MJ, Fontúrbel FE (2020) Meta-analysis of the differential effects of habitat fragmentation and degradation on plant genetic diversity. Conserv Biol 34(3):711–720
Goudet J (1995) FSTAT. (Version 2.9.3.2.): a computer program to calculate F-statistics. J Hered 86:485–486
Hamann A (2004) Flowering and fruiting phenology of a Philippine submontane rain forest: climatic factors as proximate and ultimate causes. J Ecol 92(1):24–31
Hardy O, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Izoton KB, Thomaz LD, Garbin ML, Pierre PMO (2021) Meiotic behavior and diploidy in Schinus terebinthifolia (Anacardiaceae). Rodriguésia. https://doi.org/10.1590/2175-7860202172120
Jesus BC, Gomes LJ (2013) Importância socioeconômica In: Gomes LJ, Silva-Mann R, Mattos PP, Rabbani ARC (eds) Pensando a biodiversidade: aroeira (Schinus terebinthifolia Raddi). Editora da UFS, São Cristóvão, pp 36–53
Kageyama P, Gandara FB (2004) Recuperação de áreas ciliares. In: Rodrigues RR, Leitão Filho HF (eds) Matas ciliares: Conservação e recuperação. FAPESP, São Paulo, pp 249–269
Lenzi M, Orth AI (2004) Fenologia reprodutiva, morfologia e biologia floral de Schinus terebinthifolia Raddi (Anacardeaceae), em restinga da Ilha de Santa Catarina, Brasil. Biotemas 17:67–89
Lilian M, Bastien C, José JJ, Benayas JMR, Marie-Lise B, Carolina MR, Alday JG, Jaunatre R, Dutoit T, Buisson E, Mench M, Francisco AC (2021) Conceptual and methodological issues in estimating the success of ecological restoration. Ecol Indic 123:107362
Loiselle BA et al (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Evol Syst 15:65–95
Martins LAR, Lorenzoni RM, Pereira Júnior RM, Miranda FDD, Fontes MMP, Carrijo TT, Soares TCB (2021) Genetic diversity and structure of Dorstenia elata (Moraceae) in an Atlantic Forest remnant. Rodriguésia. https://doi.org/10.1590/2175-7860202172081
Mesas A, Cuéllar-Soto E, Romero K, Zegers T, Varas V, González BA, Johnson WE, Marín JC (2021) Assessing patterns of genetic diversity and connectivity among guanacos (Lama guanicoe) in the Bolivian Chaco: implications for designing management strategies. Stud Neotrop Fauna Environ. https://doi.org/10.1080/01650521.2021.1914294
Mijangos JL et al (2015) Contribution of genetics to ecological restoration. Mol Ecol 24:22–37
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nienhuis J, Tivang J, Skroch P, dos Santos JB (1995) Genetic relationships among cultivars and landraces of lima bean (Phaseolus lunatus L.) as measured by RAPD markers. J Am Soc Hortic Sci 120(2):300–306
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Rodrigues R, Brancalion P, Isernhagen I (2009) Pacto pela restauração da Mata Atlântica: referencial dos conceitos e ações de restauração florestal. LERF/ESALQ, São Paulo
Rodrigues RR et al (2011) Large-scale ecological restoration of high-diversity tropical forests in SE Brazil. For Ecol Manag 261:1605–1613
Sebbenn AM (2002) Number of mother tree and genetics concepts in the collection of seeds for reforestation native species. Rev Inst Flor 14:115–132
Sebbenn AM (2006) Sistemas de reprodução em espécies tropicais e suas implicações para a seleção de árvores matrizes para reflorestamentos ambientais. In: Higa AR, Silva LD (coord) Pomar de sementes de espécies florestais nativas. FUPEF, Curitiba, pp 93–138
Sebbenn AM, Seoane CES (2005) Estimative of inbreeding effective size by genetic markers. Rev Árv 29:1–17
Sebbenn AM, Kageyama PY, Vencovsky R (2003) In situ genetic conservation and number of tree for seed collect in Genipa americana L. population. Sci For 63:13–22
Sergipe (2014) Secretaria de Estado do Meio Ambiente e dos Recursos Hídricos. Diagnóstico florestal de Sergipe. Aracaju
Silva TA, Pinto LVA (2009) Identification of mother trees of six allogamy species in a forest fragment aiming to the production of seedlings with genetic variability. Rev Agrogeoambient 1:56–62
SOS Mata Atlântica. Relatório anual 2016. São Paulo, 2016. https://www.sosma.org.br/wp-content/uploads/2013/05/AF_RA_SOSMA_2016_web.pdf. Accessed 14 Dezember 2017
Sujii PS et al (2017) Recovery of genetic diversity levels of a Neotropical tree in Atlantic Forest restoration plantations. Biol Conserv 211:110–116
Sujii PS, Tambarussi EV, Grando C, de Aguiar SE, Viana JPG, Brancalion PH, Zucchi MI (2021) High gene flow through pollen partially compensates spatial limited gene flow by seeds for a Neotropical tree in forest conservation and restoration areas. Conserv Genet 22(3):383–396
Vencovsky R, Crossa J (2003) Measurements of representativeness used in genetic resources conservation and plant breeding. Crop Sci 43:1912–1921
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Williams DA, Sternberg LDSL, Hughes CR (2002) Characterization of polymorphic microsatellite loci in the invasive Brazilian pepper, Schinus terebinthifolia. Mol Ecol Notes 2:231–232
Zanini AM, Mayrinck RC, Vieira SA, de Camargo PB, Rodrigues RR (2021) The effect of ecological restoration methods on carbon stocks in the Brazilian Atlantic Forest. For Ecol Manag 481:118734
Acknowledgements
We thank the National Council for Scientific and Technological Development—Brazil (CNPq), the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES ), Federal University of Sergipe, and the Research Group on Conservation, Breeding and Management of Genetic Resources (GENAPLANT).
Funding
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Grant No. 001], Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Contributions
EMSS conducted the research and wrote the text, RSM and RAF guided the research, revised the text and contributed to the writing of the manuscript, SVAC contributed to obtaining the research data, in the writing and revision of the text. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
All the authors of this manuscript declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of Doctoral Thesis of the first author.
Rights and permissions
About this article
Cite this article
de Souza, E.M.S., Álvares-Carvalho, S.V., Ferreira, R.A. et al. Schinus terebinthifolia Raddi: a comparative framework on population genetic structure in a restored area after 12 years. Genet Resour Crop Evol 69, 2459–2467 (2022). https://doi.org/10.1007/s10722-022-01384-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-022-01384-9