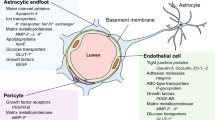
Abstract
The blood–brain barrier (BBB) is an important barrier that maintains homeostasis within the central nervous system. Brain microvascular endothelial cells are arranged to form vessel walls and express tight junctional complexes that limit the paracellular pathways of the BBB and therefore play a crucial role in ensuring brain function. These vessel walls tightly regulate the movement of ions, molecules, and cells between the blood and the brain, which protect the neural tissue from toxins and pathogens. Primary damage caused by BBB dysfunction can disrupt the expression of tight junctions, transport proteins and leukocyte adhesion molecules, leading to brain edema, disturbances in ion homeostasis, altered signaling and immune infiltration, which can lead to neuronal cell death. Various neurological diseases are known to cause BBB dysfunction, but the mechanism that causes this disorder is not clear. Recently, ferroptosis has been found to play an important role in BBB dysfunction. Ferroptosis is a new form of regulatory cell death, which is caused by the excessive accumulation of lipid peroxides and iron-dependent reactive oxygen species. This review summarizes the role of ferroptosis in BBB dysfunction and the latest progress of ferroptosis mechanism, and further discusses the influence of various factors of ferroptosis on the severity and prognosis of BBB dysfunction, which may provide better therapeutic targets for BBB dysfunction.
Graphical Abstract


Similar content being viewed by others
Data Availability
Not applicable.
Material Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- 15-LOX:
-
15-Lipoxygenase
- AA:
-
Arachidonic acid
- AC:
-
Astrocytes
- ACSL4:
-
Long-chain acyl-CoA synthetases 4
- AD:
-
Alzheimer disease
- AdA:
-
Adrenic acid
- AFIM2:
-
Apoptosis-inducing factor mitochondrial 2
- AJs:
-
Adhesion junctions
- AMP:
-
Adenosine 5′-monophosphate
- AMPK:
-
Adenosine 5′-monophosphate-activated protein kinase
- ATF4:
-
Activation of transcription factor 4
- BBB:
-
Blood–brain barrier
- BH2:
-
Dihydrobiopterin
- BH4:
-
Tetrahydrobiopterin
- BMECs:
-
Brain microvascular endothelial cells
- CNS:
-
Central nervous system
- CoQ10:
-
Coenzyme Q10
- COX:
-
Cyclooxygenase
- Cp:
-
Ceruloplasmin
- CXCL-1:
-
C-X-C motif chemokine ligand-1
- CysLTs:
-
Cysteine leukotrienes
- DFO:
-
Desferal
- DHFR:
-
Dihydrofolate reductase
- DHODH:
-
Dihydroorotate dehydrogenase
- DMT1:
-
Divalent metal transporter 1
- ESCRT-III:
-
Endosomal sorting complex required for transport
- FPN1:
-
Ferroportin 1
- FSP1:
-
Ferroptosis suppressor protein 1
- FTH1:
-
Ferritin heavy chain
- FTL:
-
Ferritin light chain
- GCH1:
-
GTP cyclohydroxylase-1
- GPX4:
-
Glutathione peroxidase 4
- GSH:
-
Glutathione
- GSSG:
-
Oxidized Glutathione
- HAMP:
-
Hepcidin antimicrobial peptide
- HNE:
-
4-Hydroxynonenal
- IRP2:
-
Iron regulatory protein 2
- l-Cys:
-
L-cysteine
- LIP:
-
Labile iron pool
- LPCAT3:
-
Lysophosphatidylcholine acyltransferase 3
- LPS:
-
Lipopolysaccharide
- LTs:
-
Leukotrienes
- MCP-1:
-
Monocyte chemoattractant protein 1
- MEG3:
-
Maternal expression gene 3
- MIP-1α:
-
Macrophage inflammatory protein-1α
- MRC:
-
Mitochondrial respiratory chain
- MS:
-
Multiple sclerosis
- NH2TP:
-
7,8-Dihydroneopterin triphosphate
- NVU:
-
Neurovascular unit
- PE:
-
Phosphatidylethanolamine
- PIC:
-
Proinflammatory cytokines
- PLOH:
-
Phospholipid alcohol
- PTP:
-
Protein tyrosine phosphatase
- PTS:
-
6-Pyruvoyl tetrahydrobiopterin synthase
- pTyr:
-
Phosphorylation
- PUFA:
-
Polyunsaturated fatty acid
- ROS:
-
Reactive oxygen species
- SEC:
-
Selenocysteine
- SECIS:
-
Selenocysteine insertion sequences
- SLC3A2:
-
Solute carrier family 3, member 2
- SLC7A11:
-
Solute carrier family 7, member 11
- SP1:
-
Specific protein 1
- SPR:
-
Sepiapterin reductase
- STEAP3:
-
Six-transmembrane epithelial antigen of the prostate
- TCA cycle:
-
Tricarboxylic acid cycle
- TF:
-
Transferrin
- TFAP2C:
-
Sec-induced transcription factor AP-2γ
- TFR1:
-
Transferrin receptor 1
- TJs:
-
Tight junctions
- ZO:
-
Zonula occludens
- α-TOH:
-
α-Tocopherol
References
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32(4):529–539. https://doi.org/10.1016/j.molcel.2008.10.021
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, Hondal RJ, Mukherjee S, Cave JW, Sagdullaev BT, Karuppagounder SS, Ratan RR (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262-1279.e1225. https://doi.org/10.1016/j.cell.2019.03.032
Archie SR, Al Shoyaib A, Cucullo L (2021) Blood–brain barrier dysfunction in CNS disorders and putative therapeutic targets: an overview. Pharmaceutics 13(11):1779. https://doi.org/10.3390/pharmaceutics13111779
Arosio P, Carmona F, Gozzelino R, Maccarinelli F, Poli M (2015) The importance of eukaryotic ferritins in iron handling and cytoprotection. Biochem J 472(1):1–15. https://doi.org/10.1042/bj20150787
Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE (2007) Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci 27(1):111–123. https://doi.org/10.1523/jneurosci.4770-06.2007
Bai T, Liang R, Zhu R, Wang W, Zhou L, Sun Y (2020) MicroRNA-214-3p enhances erastin-induced ferroptosis by targeting ATF4 in hepatoma cells. J Cell Physiol 235(7–8):5637–5648. https://doi.org/10.1002/jcp.29496
Ballerini P, Di Iorio P, Ciccarelli R, Caciagli F, Poli A, Beraudi A, Buccella S, D’Alimonte I, D’Auro M, Nargi E, Patricelli P, Visini D, Traversa U (2005) P2Y1 and cysteinyl leukotriene receptors mediate purine and cysteinyl leukotriene co-release in primary cultures of rat microglia. Int J Immunopathol Pharmacol 18(2):255–268. https://doi.org/10.1177/039463200501800208
Barnham KJ, Bush AI (2014) Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev 43(19):6727–6749. https://doi.org/10.1039/c4cs00138a
Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G (2010) Heme degradation and vascular injury. Antioxid Redox Signal 12(2):233–248. https://doi.org/10.1089/ars.2009.2822
Bersuker K, Peterson CWH, To M, Sahl SJ, Savikhin V, Grossman EA, Nomura DK, Olzmann JA (2018) A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Dev Cell 44(1):97-112.e117. https://doi.org/10.1016/j.devcel.2017.11.020
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692. https://doi.org/10.1038/s41586-019-1705-2
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110. https://doi.org/10.1016/j.biopha.2015.07.025
Black KL, Hoff JT (1985) Leukotrienes increase blood-brain barrier permeability following intraparenchymal injections in rats. Ann Neurol 18(3):349–351. https://doi.org/10.1002/ana.410180313
Blais V, Turrin NP, Rivest S (2005) Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. J Neurochem 95(6):1563–1574. https://doi.org/10.1111/j.1471-4159.2005.03480.x
Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y (2016) Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci 41(3):274–286. https://doi.org/10.1016/j.tibs.2015.11.012
Brandes RP, Weissmann N, Schröder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76:208–226. https://doi.org/10.1016/j.freeradbiomed.2014.07.046
Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA (2007) Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther 323(2):488–498. https://doi.org/10.1124/jpet.107.127035
Cao JY, Dixon SJ (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73(11–12):2195–2209. https://doi.org/10.1007/s00018-016-2194-1
Capaldo CT, Nusrat A (2009) Cytokine regulation of tight junctions. Biochim Biophys Acta 1788(4):864–871. https://doi.org/10.1016/j.bbamem.2008.08.027
Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, Tsai AL, Zweier JL (2010) Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry 49(14):3129–3137. https://doi.org/10.1021/bi9016632
Chen X, Li J, Kang R, Klionsky DJ, Tang D (2021) Ferroptosis: machinery and regulation. Autophagy 17(9):2054–2081. https://doi.org/10.1080/15548627.2020.1810918
Cho J, Teshigawara R, Kameda M, Yamaguchi S, Tada T (2019) Nucleus-localized adiponectin is survival gatekeeper through miR-214-mediated AIFM2 regulation. Genes Cells 24(2):126–138. https://doi.org/10.1111/gtc.12658
Chodobski A, Zink BJ, Szmydynger-Chodobska J (2011) Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2(4):492–516. https://doi.org/10.1007/s12975-011-0125-x
Ciccarelli R, D’Alimonte I, Santavenere C, D’Auro M, Ballerini P, Nargi E, Buccella S, Nicosia S, Folco G, Caciagli F, Di Iorio P (2004) Cysteinyl-leukotrienes are released from astrocytes and increase astrocyte proliferation and glial fibrillary acidic protein via cys-LT1 receptors and mitogen-activated protein kinase pathway. Eur J Neurosci 20(6):1514–1524. https://doi.org/10.1111/j.1460-9568.2004.03613.x
Conrad M, Friedmann Angeli JP (2015) Glutathione peroxidase 4 (Gpx4) and ferroptosis: what’s so special about it? Mol Cell Oncol 2(3):e995047. https://doi.org/10.4161/23723556.2014.995047
Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, Pierson Y, McNeill E, Longhi MS, Turnes BL, Kreslavsky T, Kogler M, Hoffmann D, Ticevic M, da Luz SD, Tortola L, Cikes D, Jais A, Rangachari M, Rao S, Paolino M, Novatchkova M, Aichinger M, Barrett L, Latremoliere A, Wirnsberger G, Lametschwandtner G, Busslinger M, Zicha S, Latini A, Robson SC, Waisman A, Andrews N, Costigan M, Channon KM, Weiss G, Kozlov AV, Tebbe M, Johnsson K, Woolf CJ, Penninger JM (2018) The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 563(7732):564–568. https://doi.org/10.1038/s41586-018-0701-2
Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D (2020) AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun 523(4):966–971. https://doi.org/10.1016/j.bbrc.2020.01.066
Dalvi S, Nguyen HH, On N, Mitchell RW, Aukema HM, Miller DW, Hatch GM (2015) Exogenous arachidonic acid mediates permeability of human brain microvessel endothelial cells through prostaglandin E2 activation of EP3 and EP4 receptors. J Neurochem 135(5):867–879. https://doi.org/10.1111/jnc.13117
DeGregorio-Rocasolano N, Martí-Sistac O, Gasull T (2019) Deciphering the iron side of stroke: neurodegeneration at the crossroads between iron dyshomeostasis, excitotoxicity, and ferroptosis. Front Neurosci 13:85. https://doi.org/10.3389/fnins.2019.00085
Ding H, Yan CZ, Shi H, Zhao YS, Chang SY, Yu P, Wu WS, Zhao CY, Chang YZ, Duan XL (2011) Hepcidin is involved in iron regulation in the ischemic brain. PLoS ONE 6(9):e25324. https://doi.org/10.1371/journal.pone.0025324
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10(1):9–17. https://doi.org/10.1038/nchembio.1416
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B III, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575(7784):693–698. https://doi.org/10.1038/s41586-019-1707-0
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62(6):649–671. https://doi.org/10.1016/s0301-0082(99)00060-x
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Elguindy MM, Nakamaru-Ogiso E (2015) Apoptosis-inducing factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH: ubiquinone oxidoreductases (NDH-2). J Biol Chem 290(34):20815–20826. https://doi.org/10.1074/jbc.M115.641498
Engelhardt S, Al-Ahmad AJ, Gassmann M, Ogunshola OO (2014) Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J Cell Physiol 229(8):1096–1105. https://doi.org/10.1002/jcp.24544
Ernster L, Dallner G (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271(1):195–204. https://doi.org/10.1016/0925-4439(95)00028-3
Evstatiev R, Gasche C (2012) Iron sensing and signalling. Gut 61(6):933–952. https://doi.org/10.1136/gut.2010.214312
Fang KM, Cheng FC, Huang YL, Chung SY, Jian ZY, Lin MC (2013) Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol Trace Elem Res 152(1):66–74. https://doi.org/10.1007/s12011-012-9596-1
Filomeni G, Rotilio G, Ciriolo MR (2002) Cell signalling and the glutathione redox system. Biochem Pharmacol 64(5–6):1057–1064. https://doi.org/10.1016/s0006-2952(02)01176-0
Florean C, Song S, Dicato M, Diederich M (2019) Redox biology of regulated cell death in cancer: a focus on necroptosis and ferroptosis. Free Radic Biol Med 134:177–189. https://doi.org/10.1016/j.freeradbiomed.2019.01.008
Friedmann Angeli JP, Conrad M (2018) Selenium and GPX4, a vital symbiosis. Free Radic Biol Med 127:153–159. https://doi.org/10.1016/j.freeradbiomed.2018.03.001
Gan L, Johnson JA (2014) Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta 1842(8):1208–1218. https://doi.org/10.1016/j.bbadis.2013.12.011
Ghasemloo E, Oryan S, Bigdeli MR, Mostafavi H, Eskandari M (2021) The neuroprotective effect of MicroRNA-149-5p and coenzymeQ10 by reducing levels of inflammatory cytokines and metalloproteinases following focal brain ischemia in rats. Brain Res Bull 169:205–213. https://doi.org/10.1016/j.brainresbull.2021.01.013
Ghosh A, Chen F, Thakur A, Hong H (2016) Cysteinyl leukotrienes and their receptors: emerging therapeutic targets in central nervous system disorders. CNS Neurosci Ther 22(12):943–951. https://doi.org/10.1111/cns.12596
Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD, Matthews RB, Frey WH II, Panter SS (2009) Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther 330(3):679–686. https://doi.org/10.1124/jpet.108.149807
Harris TJ (2012) An introduction to adherens junctions: from molecular mechanisms to tissue development and disease. Subcell Biochem 60:1–5. https://doi.org/10.1007/978-94-007-4186-7_1
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185. https://doi.org/10.1124/pr.57.2.4
Hiroki O (2012) Evolution of the cadherin-catenin complex. Subcell Biochem 60:9–35. https://doi.org/10.1007/978-94-007-4186-7_2
Huang S-F, Othman A, Koshkin A, Fischer S, Fischer D, Zamboni N, Ono K, Sawa T, Ogunshola OO (2020a) Astrocyte glutathione maintains endothelial barrier stability. Redox Biol 34:101576–101576. https://doi.org/10.1016/j.redox.2020.101576
Huang SF, Othman A, Koshkin A, Fischer S, Fischer D, Zamboni N, Ono K, Sawa T, Ogunshola OO (2020b) Astrocyte glutathione maintains endothelial barrier stability. Redox Biol 34:101576. https://doi.org/10.1016/j.redox.2020.101576
Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10(11):1369–1376. https://doi.org/10.1038/nn2003
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34(2):145–169. https://doi.org/10.1016/s0891-5849(02)01197-8
Imai T, Iwata S, Hirayama T, Nagasawa H, Nakamura S, Shimazawa M, Hara H (2019a) Intracellular Fe2+ accumulation in endothelial cells and pericytes induces blood–brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci Rep 9(1):6228–6228. https://doi.org/10.1038/s41598-019-42370-z
Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arnér ESJ, Fradejas-Villar N, Schweizer U, Zischka H, Friedmann Angeli JP, Conrad M (2018) Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172(3):409-422.e421. https://doi.org/10.1016/j.cell.2017.11.048
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood–brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171. https://doi.org/10.1016/j.pneurobio.2017.10.001
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90. https://doi.org/10.1038/nchembio.2238
Kale G, Naren AP, Sheth P, Rao RK (2003) Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun 302(2):324–329. https://doi.org/10.1016/s0006-291x(03)00167-0
Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K (1995) Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res 23(5):419–430. https://doi.org/10.3109/10715769509065263
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, Brandner S, Daniels JD, Schmitt-Kopplin P, Hauck SM, Stockwell BR, Hadian K, Schick JA (2020) GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci 6(1):41–53. https://doi.org/10.1021/acscentsci.9b01063
Lan X, Han X, Li Q, Wang J (2017) (-)-Epicatechin, a natural flavonoid compound, protects astrocytes against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways. Mol Neurobiol 54(10):7898–7907. https://doi.org/10.1007/s12035-016-0271-y
Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, Nakada D, Stockwell BR, Gan B (2020) Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol 22(2):225–234. https://doi.org/10.1038/s41556-020-0461-8
Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, Gan B (2020) The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res 30(2):146–162. https://doi.org/10.1038/s41422-019-0263-3
Levine RA, Kapatos G, Kaufman S, Milstien S (1990) Immunological evidence for the requirement of sepiapterin reductase for tetrahydrobiopterin biosynthesis in brain. J Neurochem 54(4):1218–1224. https://doi.org/10.1111/j.1471-4159.1990.tb01951.x
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P (2013) The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18(5):522–555. https://doi.org/10.1089/ars.2011.4391
Liu H, Hua Y, Keep RF, Xi G (2019) Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury. Transl Stroke Res 10(1):112–119. https://doi.org/10.1007/s12975-018-0669-0
Lu CJ, Guo YZ, Zhang Y, Yang L, Chang Y, Zhang JW, Jing L, Zhang JZ (2017) Coenzyme Q10 ameliorates cerebral ischemia reperfusion injury in hyperglycemic rats. Pathol Res Pract 213(9):1191–1199. https://doi.org/10.1016/j.prp.2017.06.005
Lucas K, Morris G, Anderson G, Maes M (2015) The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets 14(7):838–854. https://doi.org/10.2174/1871527314666150317224645
Ma B, Day JP, Phillips H, Slootsky B, Tolosano E, Doré S (2016) Deletion of the hemopexin or heme oxygenase-2 gene aggravates brain injury following stroma-free hemoglobin-induced intracerebral hemorrhage. J Neuroinflammation 13:26. https://doi.org/10.1186/s12974-016-0490-1
Magtanong L, Dixon SJ (2018) Ferroptosis and brain injury. Dev Neurosci 40(5–6):382–395. https://doi.org/10.1159/000496922
Marschallinger J, Schäffner I, Klein B, Gelfert R, Rivera FJ, Illes S, Grassner L, Janssen M, Rotheneichner P, Schmuckermair C, Coras R, Boccazzi M, Chishty M, Lagler FB, Renic M, Bauer HC, Singewald N, Blümcke I, Bogdahn U, Couillard-Despres S, Lie DC, Abbracchio MP, Aigner L (2015) Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat Commun 6:8466. https://doi.org/10.1038/ncomms9466
Martin W, Russell MJ (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc Lond B Biol Sci 358(1429):59–83. https://doi.org/10.1098/rstb.2002.1183
McCarthy RC, Kosman DJ (2015) Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci 72(4):709–727. https://doi.org/10.1007/s00018-014-1771-4
Moens AL, Kass DA (2006) Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26(11):2439–2444. https://doi.org/10.1161/01.ATV.0000243924.00970.cb
Moos T, Morgan EH (2000) Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol 20(1):77–95. https://doi.org/10.1023/a:1006948027674
Morris G, Berk M (2015) The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 13:68. https://doi.org/10.1186/s12916-015-0310-y
Morris G, Maes M (2014) Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol 12(2):168–185. https://doi.org/10.2174/1570159x11666131120224653
Morris G, Berk M, Galecki P, Walder K, Maes M (2016) The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol Neurobiol 53(2):1195–1219. https://doi.org/10.1007/s12035-015-9090-9
Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D (2013) Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation 10:142. https://doi.org/10.1186/1742-2094-10-142
Nigam S, Schewe T (2000) Phospholipase A(2)s and lipid peroxidation. Biochim Biophys Acta 1488(1–2):167–181. https://doi.org/10.1016/s1388-1981(00)00119-0
Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L (2012) Mechanisms of mammalian iron homeostasis. Biochemistry 51(29):5705–5724. https://doi.org/10.1021/bi300752r
Park UJ, Lee YA, Won SM, Lee JH, Kang SH, Springer JE, Lee YB, Gwag BJ (2011) Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol 121(4):459–473. https://doi.org/10.1007/s00401-010-0785-8
Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU (1999) 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal 1(3):255–284. https://doi.org/10.1089/ars.1999.1.3-255
Pierzynowska K, Rintz E, Gaffke L, Węgrzyn G (2021) Ferroptosis and its modulation by autophagy in light of the pathogenesis of lysosomal storage diseases. Cells 10(2):365. https://doi.org/10.3390/cells10020365
Rahman I, Kinnula VL (2012) Strategies to decrease ongoing oxidant burden in chronic obstructive pulmonary disease. Expert Rev Clin Pharmacol 5(3):293–309. https://doi.org/10.1586/ecp.12.16
Rand D, Ravid O, Atrakchi D, Israelov H, Bresler Y, Shemesh C, Omesi L, Liraz-Zaltsman S, Gosselet F, Maskrey TS, Beeri MS, Wipf P, Cooper I (2021) Endothelial iron homeostasis regulates blood–brain barrier integrity via the HIF2α-Ve-cadherin pathway. Pharmaceutics 13(3):311. https://doi.org/10.3390/pharmaceutics13030311
Rao R (2008) Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci 13:7210–7226. https://doi.org/10.2741/3223
Ravet K, Pilon M (2013) Copper and iron homeostasis in plants: the challenges of oxidative stress. Antioxid Redox Signal 19(9):919–932. https://doi.org/10.1089/ars.2012.5084
Salehpour F, Farajdokht F, Mahmoudi J, Erfani M, Farhoudi M, Karimi P, Rasta SH, Sadigh-Eteghad S, Hamblin MR, Gjedde A (2019) Photobiomodulation and coenzyme Q(10) treatments attenuate cognitive impairment associated with model of transient global brain ischemia in artificially aged mice. Front Cell Neurosci 13:74. https://doi.org/10.3389/fncel.2019.00074
Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE (2007) Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev 56(2):322–330. https://doi.org/10.1016/j.brainresrev.2007.07.005
Seibt TM, Proneth B, Conrad M (2019) Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 133:144–152. https://doi.org/10.1016/j.freeradbiomed.2018.09.014
Shang Y, Luo M, Yao F, Wang S, Yuan Z, Yang Y (2020) Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal 72:109633. https://doi.org/10.1016/j.cellsig.2020.109633
Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC (2011) Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol 90(4):323–332. https://doi.org/10.1016/j.ejcb.2010.10.013
Shindou H, Shimizu T (2009) Acyl-CoA: lysophospholipid acyltransferases. J Biol Chem 284(1):1–5. https://doi.org/10.1074/jbc.R800046200
Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K (2020) Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16(12):1351–1360. https://doi.org/10.1038/s41589-020-0613-y
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31(2):107–125. https://doi.org/10.1038/s41422-020-00441-1
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121. https://doi.org/10.3233/jad-161088
Valdovinos-Flores C, Gonsebatt ME (2012) The role of amino acid transporters in GSH synthesis in the blood–brain barrier and central nervous system. Neurochem Int 61(3):405–414. https://doi.org/10.1016/j.neuint.2012.05.019
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL (2017) Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547(7664):453–457. https://doi.org/10.1038/nature23007
Wan J, Ren H, Wang J (2019a) Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol 4(2):93–95. https://doi.org/10.1136/svn-2018-000205
Wan W, Cao L, Kalionis B, Murthi P, Xia S, Guan Y (2019b) Iron deposition leads to hyperphosphorylation of tau and disruption of insulin signaling. Front Neurol 10:607–607. https://doi.org/10.3389/fneur.2019.00607
Wang X, Li GJ, Zheng W (2008a) Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: effect of manganese exposure. Exp Biol Med (maywood) 233(12):1561–1571. https://doi.org/10.3181/0803-rm-104
Wang X, Miller DS, Zheng W (2008b) Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol Appl Pharmacol 230(2):167–174. https://doi.org/10.1016/j.taap.2008.02.024
Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W (2019) CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569(7755):270–274. https://doi.org/10.1038/s41586-019-1170-y
Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J (2019) Ferroptosis and its role in diverse brain diseases. Mol Neurobiol 56(7):4880–4893. https://doi.org/10.1007/s12035-018-1403-3
Weiss N, Miller F, Cazaubon S, Couraud PO (2009) The blood–brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 1788(4):842–857. https://doi.org/10.1016/j.bbamem.2008.10.022
Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA, Amoscato AA, Mikulska-Ruminska K, Shrivastava IH, Kenny EM, Yang Q, Rosenbaum JC, Sparvero LJ, Emlet DR, Wen X, Minami Y, Qu F, Watkins SC, Holman TR, VanDemark AP, Kellum JA, Bahar I, Bayır H, Kagan VE (2017) PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171(3):628-641.e626. https://doi.org/10.1016/j.cell.2017.09.044
Wong V (1997) Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol 273(6):C1859-1867. https://doi.org/10.1152/ajpcell.1997.273.6.C1859
Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, Jiang JY (2019) Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther 25(4):465–475. https://doi.org/10.1111/cns.13069
Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T (2008) Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol 172(2):521–533. https://doi.org/10.2353/ajpath.2008.070076
Yan H-F, Tuo Q-Z, Yin Q-Z, Lei P (2020) The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool Res 41(3):220–230. https://doi.org/10.24272/j.issn.2095-8137.2020.042
Yan BC, Cao J, Liu J, Gu Y, Xu Z, Li D, Gao L (2021) Dietary Fe3O4 nanozymes prevent the injury of neurons and blood–brain barrier integrity from cerebral ischemic stroke. ACS Biomater Sci Eng 7(1):299–310. https://doi.org/10.1021/acsbiomaterials.0c01312
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15(3):234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26(3):165–176. https://doi.org/10.1016/j.tcb.2015.10.014
Yasothornsrikul S, Aaron W, Toneff T, Hook VY (1999) Evidence for the proenkephalin processing enzyme prohormone thiol protease (PTP) as a multicatalytic cysteine protease complex: activation by glutathione localized to secretory vesicles. Biochemistry 38(23):7421–7430. https://doi.org/10.1021/bi990239w
Yu H, Guo P, Xie X, Wang Y, Chen G (2017) Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med 21(4):648–657. https://doi.org/10.1111/jcmm.13008
Zhang XY, Wang XR, Xu DM, Yu SY, Shi QJ, Zhang LH, Chen L, Fang SH, Lu YB, Zhang WP, Wei EQ (2013) HAMI 3379, a CysLT2 receptor antagonist, attenuates ischemia-like neuronal injury by inhibiting microglial activation. J Pharmacol Exp Ther 346(2):328–341. https://doi.org/10.1124/jpet.113.203604
Zheng W, Monnot AD (2012) Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol Ther 133(2):177–188. https://doi.org/10.1016/j.pharmthera.2011.10.006
Ziai WC (2013) Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke 44(6 Suppl 1):S74-78. https://doi.org/10.1161/strokeaha.111.000662
Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA, Ratan RR (2017) Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 48(4):1033–1043. https://doi.org/10.1161/strokeaha.116.015609
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. https://doi.org/10.1016/j.neuron.2008.01.003
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorg Biochem 195:120–129. https://doi.org/10.1016/j.jinorgbio.2019.03.013
Acknowledgements
This work was supported by National Natural Science Foundation of China and Natural Science Foundation of Hunan Province.
Funding
This work was supported by National Natural Science Foundation of China (Grant Nos. 81571880; 81373147; 30901555; 30972870; 81360080; 82172147) and Natural Science Foundation of Hunan Province (Grant Nos. 2016JJ2157; 2021JJ30900).
Author information
Authors and Affiliations
Contributions
YL and JZ designed and directed the project. YZ wrote the manuscript. YZ, YL, YX, KL, HQ, QX and JZ collected materials. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Liu, Y., Xu, Y. et al. The Role of Ferroptosis in Blood–Brain Barrier Injury. Cell Mol Neurobiol 43, 223–236 (2023). https://doi.org/10.1007/s10571-022-01197-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01197-5