Abstract
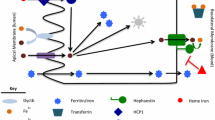
There are two barriers for iron entry into the brain: (1) the brain–cerebrospinal fluid (CSF) barrier and (2) the blood–brain barrier (BBB). Here, we review the literature on developmental iron accumulation by the brain, focusing on the transport of iron through the brain microvascular endothelial cells (BMVEC) of the BBB. We review the iron trafficking proteins which may be involved in the iron flux across BMVEC and discuss the plausible mechanisms of BMVEC iron uptake and efflux. We suggest a model for how BMVEC iron uptake and efflux are regulated and a mechanism by which the majority of iron is trafficked across the developing BBB under the direct guidance of neighboring astrocytes. Thus, we place brain iron uptake in the context of the neurovascular unit of the adult brain. Last, we propose that BMVEC iron is involved in the aggregation of amyloid-β peptides leading to the progression of cerebral amyloid angiopathy which often occurs prior to dementia and the onset of Alzheimer’s disease.




Similar content being viewed by others
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- APP:
-
Amyloid-β precursor protein
- sAPP:
-
Soluble β-secretase-cleaved APP
- Aβ:
-
Amyloid-β
- BBB:
-
Blood–brain barrier
- BMVEC:
-
Brain microvascular endothelial cells
- CNS:
-
Central nervous system
- Cp:
-
Ceruloplasmin
- CSF:
-
Cerebrospinal fluid
- CTFβ:
-
β-Carboxyl-terminal fragment
- Dcytb:
-
Duodenal cytochrome b
- DMT1:
-
Divalent metal transporter 1
- E°:
-
Electrochemical potential
- Fpn:
-
Ferroportin
- GPI:
-
Glycosylphosphatidylinositol-anchor
- hBMVEC:
-
Human brain microvascular endothelial cell line
- H-Ferritin:
-
Heavy chain ferritin
- Hp:
-
Hephaestin
- IRE:
-
Iron-responsive element
- IRP:
-
Iron regulatory protein
- L-Ferritin:
-
Light chain ferritin
- P#:
-
Postnatal day #
- sCp:
-
Soluble ceruloplasmin
- SDR2:
-
Stromal cell-derived receptor 2
- Steap:
-
Six transmembrane epithelial antigen of the prostate
- Tf:
-
Transferrin
- TfR:
-
Transferrin receptor
- Zip8:
-
Zrt/Irt-like protein 8
- Zip14:
-
Zrt/Irt-like protein 14
- Zp:
-
Zyklopen
References
Cheepsunthorn P, Palmer C, Connor JR (1998) Cellular distribution of ferritin subunits in postnatal rat brain. J Comp Neurol 400(1):73–86
Todorich B et al (2009) Oligodendrocytes and myelination: the role of iron. Glia 57(5):467–478
Salvador GA (2010) Iron in neuronal function and dysfunction. BioFactors 36(2):103–110
Madsen E, Gitlin JD (2007) Copper and iron disorders of the brain. Annu Rev Neurosci 30(1):317–337
Rivera-Mancía S et al (2010) The transition metals copper and iron in neurodegenerative diseases. Chem Biol Interact 186(2):184–199
Salazar J et al (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci USA 105(47):18578–18583
Rouault TA (2013) Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 14(8):551–564
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7(1):41–53
Rouault TA, Cooperman S (2006) Brain iron metabolism. Semin Pediatr Neurol 13(3):142–148
Xu J, Ling EA (1994) Studies of the ultrastructure and permeability of the blood–brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J Anat 184(Pt 2):227–237
Fisher J et al (2007) Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol 293(2):C641–C649
Oide T et al (2006) Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia. Neuropathol Appl Neurobiol 32(2):170–176
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5(5):347–360
McCarthy RC, Kosman DJ (2014) Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS ONE 9(2):e89003
Greco TM et al (2010) Quantitative mass spectrometry-based proteomics reveals the dynamic range of primary mouse astrocyte protein secretion. J Proteome Res 9(5):2764–2774
Aisen P, Leibman A, Zweier J (1978) Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem 253(6):1930–1937
Byrne SL et al (2010) The unique kinetics of iron release from transferrin: the role of receptor, lobe–lobe interactions, and salt at endosomal pH. J Mol Biol 396(1):130–140
Dhungana S et al (2004) Redox properties of human transferrin bound to its receptor. Biochemistry 43(1):205–209
Weaver KD et al (2010) Role of citrate and phosphate anions in the mechanism of iron(III) sequestration by ferric binding protein: kinetic studies of the formation of the holoprotein of wild-type FbpA and its engineered mutants. Biochemistry 49(29):6021–6032
Bloch B et al (1985) Transferrin gene expression visualized in oligodendrocytes of the rat brain by using in situ hybridization and immunohistochemistry. Proc Natl Acad Sci 82(19):6706–6710
De los Monteros AE et al (1990) Transferrin gene expression and secretion by rat brain cells in vitro. J Neurosci Res 25(4):576–580
Zahs KR, Bigornia V, Deschepper CF (1993) Characterization of “plasma proteins” secreted by cultured rat macroglial cells. Glia 7(2):121–133
Connor JR, Fine RE (1986) The distribution of transferrin immunoreactivity in the rat central nervous system. Brain Res 368(2):319–328
Koeller DM et al (1989) A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci USA 86(10):3574–3578
Casey JL et al (1988) Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science 240(4854):924–928
Erlitzki R, Long JC, Theil EC (2002) Multiple, conserved iron-responsive elements in the 3′-untranslated region of transferrin receptor mRNA enhance binding of iron regulatory protein 2. J Biol Chem 277(45):42579–42587
Mullner EW, Kuhn LC (1988) A region in the 3′ untranslated region mediates iron dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell 53:815–825
Raub TJ, Newton CR (1991) Recycling kinetics and transcytosis of transferrin in primary cultures of bovine brain microvessel endothelial cells. J Cell Physiol 149(1):141–151
Burdo J et al (2001) Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 66(6):1198–1207
Moos T, Morgan E (2000) Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol 20(1):77–95
Moos T et al (2006) Brain capillary endothelial cells mediate iron transport into the brain by segregating iron from transferrin without the involvement of divalent metal transporter 1. J Neurochem 98(6):1946–1958
Rothenberger S et al (1996) Coincident expression and distribution of melanotransferrin and transferrin receptor in human brain capillary endothelium. Brain Res 712(1):117–121
Yang W et al (2011) Transient expression of iron transport proteins in the capillary of the developing rat brain. Cell Mol Neurobiol 31(1):93–99
Siddappa AJM et al (2002) Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res 68(6):761–775
Taylor EM, Crowe A, Morgan EH (1991) Transferrin and iron uptake by the brain: effects of altered iron status. J Neurochem 57(5):1584–1592
McCarthy RC, Kosman DJ (2013) Ferroportin and exocytoplasmic ferroxidase activity are required for brain microvascular endothelial cell iron efflux. J Biol Chem 288(24):17932–17940
Ohgami RS et al (2005) Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37(11):1264–1269
Ohgami RS et al (2006) The Steap proteins are metalloreductases. Blood 108(4):1388–1394
Knutson MD (2007) Steap proteins: implications for iron and copper metabolism. Nutr Rev 65(7):335–340
McCarthy RC, Kosman DJ (2012) Mechanistic analysis of iron accumulation by endothelial cells of the BBB. Biometals 25(4):665–675
Ohgami RS et al (2005) Nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood 106(10):3625–3631
McKie AT et al (2001) An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291(5509):1755–1759
Wyman S et al (2008) Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett 582(13):1901–1906
Latunde-Dada GO, Simpson RJ, McKie AT (2008) Duodenal cytochrome b expression stimulates iron uptake by human intestinal epithelial cells. J Nutr 138(6):991–995
Turi JL et al (2006) Duodenal cytochrome b: a novel ferrireductase in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 291(2):L272–L280
Tulpule K et al (2010) Uptake of ferrous iron by cultured rat astrocytes. J Neurosci Res 88(3):563–571
Jeong SY, David S (2003) Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem 278(29):27144–27148
Loke SY et al (2013) Expression and localization of duodenal cytochrome b in the rat hippocampus after kainate-induced excitotoxicity. Neuroscience 245:179–190
Lane DJ, Lawen A (2008) Non-transferrin iron reduction and uptake are regulated by transmembrane ascorbate cycling in K562 cells. J Biol Chem 283(19):12701–12708
Lane DJ et al (2010) Two routes of iron accumulation in astrocytes: ascorbate-dependent ferrous iron uptake via the divalent metal transporter (DMT1) plus an independent route for ferric iron. Biochem J 432(1):123–132
Oates PS et al (2000) Gene expression of divalent metal transporter 1 and transferrin receptor in duodenum of Belgrade rats. Am J Physiol Gastrointest Liver Physiol 278(6):G930–G936
Fleming RE et al (1999) Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci USA 96(6):3143–3148
Trinder D et al (2000) Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut 46(2):270–276
Canonne-Hergaux F et al (2000) The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood 96(12):3964–3970
Gunshin H et al (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388(6641):482–488
Garrick MD et al (2006) DMT1: which metals does it transport? Biol Res 39:79–85
Knöpfel M, Smith C, Solioz M (2005) ATP-driven copper transport across the intestinal brush border membrane. Biochem Biophys Res Commun 330(3):645–652
Garrick MD, Kuo HC, Vargas F, Singleton S, Zhao L, Smith JJ, Paradkar P, Roth JA, Garrick LM (2006) Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochem J 398(3):539–545
Worthington MT et al (2000) Functional properties of transfected human DMT1 iron transporter. Am J Physiol Gastrointest Liver Physiol 279(6):G1265–G1273
McEwan GTA et al (1988) The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine. Proc R Soc Lond B Biol Sci 234(1275):219–237
Quigley EM, Turnberg LA (1992) Studies of luminal and mucosal pH in reflux esophagitis and antral gastritis. Dig Dis 10(3):134–143
Conrad ME et al (2000) Separate pathways for cellular uptake of ferric and ferrous iron. Am J Physiol Gastrointest Liver Physiol 279(4):G767–G774
Mackenzie B et al (2006) Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflügers Arch 451(4):544–558
Skjørringe T, Møller LB, Moos T (2012) Impairment of interrelated iron- and copper homeostatic mechanisms in brain contributes to the pathogenesis of neurodegenerative disorders. Front Pharmacol 3:169
Fleming MD et al (1998) Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95(3):1148–1153
Farcich EA, Morgan EH (1992) Diminished iron acquisition by cells and tissues of Belgrade laboratory rats. Am J Physiol 262(2):R220–R224
Burdo JR et al (1999) Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience 93(3):1189–1196
Taylor KM et al (2005) Structure–function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett 579(2):427–432
Liuzzi JP et al (2006) Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103(37):13612–13617
Pinilla-Tenas JJ et al (2011) Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol Cell Physiol 301(4):C862–C871
Zhao N et al (2010) ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem 285(42):32141–32150
Girijashanker K et al (2008) Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharm 73(5):1413–1423
Wang C-Y et al (2012) ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem 287(41):34032–34043
Kraiter DC et al (1998) A determination of the reduction potentials for diferric and C- and N-lobe monoferric transferrins at endosomal pH (5.8). Inorg Chem 37(5):964–968
Dhungana S et al (2003) Redox properties of human transferrin bound to its receptor†. Biochemistry 43(1):205–209
Byrne S, Mason A (2009) Human serum transferrin: a tale of two lobes. Urea gel and steady state fluorescence analysis of recombinant transferrins as a function of pH, time, and the soluble portion of the transferrin receptor. J Biol Inorg Chem 14(5):771–781
Nelson N, Harvey WR (1999) Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79(2):361–385
Moos T et al (2007) Iron trafficking inside the brain. J Neurochem 103(5):1730–1740
Descamps L et al (1996) Receptor-mediated transcytosis of transferrin through blood–brain barrier endothelial cells. Am J Physiol 270(4):H1149–H1158
Kintner DB et al (2000) 31P-MRS-based determination of brain intracellular and interstitial pH: its application to in vivo H+ compartmentation and cellular regulation during hypoxic/ischemic conditions. Neurochem Res 25(9):1385–1396
Bradbury MWB (1997) Transport of iron in the blood–brain–cerebrospinal fluid system. J Neurochem 69(2):443–454
Crowe A, Morgan EH (1992) Iron and transferrrin uptake by brain and cerebrospinal fluid in the rat. Brain Res 592(1–2):8–16
Manich G et al (2013) Study of the transcytosis of an anti-transferrin receptor antibody with a Fab′ cargo across the blood–brain barrier in mice. Eur J Pharm Sci. 49:556–564
Laurie GW, Leblond CP, Martin GR (1982) Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol 95(1):340–344
Meguro R et al (2008) Cellular and subcellular localizations of nonheme ferric and ferrous iron in the rat brain: a light and electron microscopic study by the perfusion-Perls and -Turnbull methods. Arch Histol Cytol 71(4):205–222
Riemer J et al (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331(2):370–375
Wolcott GH, Boyer PD (1948) A colorimetric method for the determination of citric acid in blood and plasma. J Biol Chem 172(2):729–736
Bates GW, Billups C, Saltman P (1967) The kinetics and mechanism of iron(III) exchange between chelates and transferrin. J Biol Chem 242(12):2810–2815
Königsberger L-C et al (2000) Complexation of iron(III) and iron(II) by citrate. Implications for iron speciation in blood plasma. J Inorg Biochem 78(3):175–184
Hearers AF (1971) Citrate and alpha-ketoglutarate in cerebrospinal fluid and blood. Neurology 21(10):1059
Donovan A et al (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403(6771):776–781
McKie AT et al (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5(2):299–309
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275(26):19906–19912
Mitchell CJ et al (2014) Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am J Physiol Cell Physiol 306(5):C450–C459
Madejczyk MS, Ballatori N (2012) The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta 1818(3):651–657
Yin Z et al (2010) Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem 112(5):1190–1198
De Domenico I et al (2007) Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J 26(12):2823–2831
De Domenico I et al (2007) Evidence for the multimeric structure of ferroportin. Blood 109(5):2205–2209
De Domenico I et al (2005) The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA 102(25):8955–8960
De Domenico I et al (2010) Human mutation D157G in ferroportin leads to hepcidin-independent binding of Jak2 and ferroportin down-regulation. Blood 115(14):2956–2959
Montosi G et al (2001) Autosomal-dominant hemochrom-atosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest 108(4):619–623
Liu X-B, Yang F, Haile DJ (2005) Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis 35(1):33–46
Wallace DF, Harris JM, Subramaniam VN (2010) Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am J Physiol Cell Physiol 298(1):C75–C84
Leipuviene R, Theil E (2007) The family of iron responsive RNA structures regulated by changes in cellular iron and oxygen. Cell Mol Life Sci 64(22):2945–2955
Ward DM, Kaplan J (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 1823(9):1426–1433
Zhang D-L et al (2011) Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood 118(10):2868–2877
Nemeth E et al (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306(5704):2090–2093
Qiao B et al (2012) Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab 15(6):918–924
De Domenico I et al (2008) The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab 8(2):146–156
De Domenico I et al (2007) The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18(7):2569–2578
Ross Sandra L et al (2012) Molecular mechanism of hepcidin-mediated ferroportin internalization requires ferroportin lysines, not tyrosines or JAK-STAT. Cell Metab 15(6):905–917
Preza GC et al (2013) Cellular catabolism of the iron-regulatory peptide hormone hepcidin. PLoS ONE 8(3):e58934
Kono S et al (2010) Biological effects of mutant ceruloplasmin on hepcidin-mediated internalization of ferroportin. Biochim Biophys Acta 1802(11):968–975
Zechel S, Huber-Wittmer K, von Bohlen O, Halbach (2006) Distribution of the iron-regulating protein hepcidin in the murine central nervous system. J Neurosci Res 84(4):790–800
Han O, Kim E-Y (2007) Colocalization of ferroportin-1 with hephaestin on the basolateral membrane of human intestinal absorptive cells. J Cell Biochem 101(4):1000–1010
Wu LJ-c et al (2004) Expression of the iron transporter ferroportin in synaptic vesicles and the blood–brain barrier. Brain Res 1001(1–2):108–117
Raha A et al (2013) The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuro Comms 1(1):55
Boserup M et al (2011) Heterogenous distribution of ferroportin-containing neurons in mouse brain. Biometals 24(2):357–375
Moos T, Rosengren Nielsen T (2006) Ferroportin in the postnatal rat brain: implications for axonal transport and neuronal export of iron. Semin Pediatr Neurol 13(3):149–157
Schulz K et al (2011) Iron efflux from oligodendrocytes is differentially regulated in gray and white matter. J Neurosci 31(37):13301–13311
Enerson BE, Drewes LR (2005) The rat blood–brain barrier transcriptome. J Cereb Blood Flow Metab 26(7):959–973
Fung E et al (2013) High-throughput screening of small molecules identifies hepcidin antagonists. Mol Pharm 83(3):681–690
Nittis T, Gitlin JD (2004) Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol Chem 279(24):25696–25702
Griffiths TAM, Mauk AG, MacGillivray RTA (2005) Recombinant expression and functional characterization of human hephaestin: a multicopper oxidase with ferroxidase activity. Biochemistry 44(45):14725–14731
Bento I et al (2007) Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites. Acta Crystallogr D Biol Crystallogr 63(2):240–248
Sato M, Gitlin JD (1991) Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem 266(8):5128–5134
Zaitseva I et al (1996) The X-ray structure of human serum ceruloplasmin at 3.1 Å: nature of the copper centres. J Biol Inorg Chem 1(1):15–23
Chen H et al (2010) Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J Nutr 140(10):1728–1735
Danzeisen R et al (2000) The effect of ceruloplasmin on iron release from placental (BeWo) cells; evidence for an endogenous Cu oxidase. Placenta 21(8):805–812
Danzeisen R et al (2002) Placental ceruloplasmin homolog is regulated by iron and copper and is implicated in iron metabolism. Am J Physiol Cell Physiol 282(3):C472–C478
Vulpe CD et al (1999) Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21(2):195–199
Lee S-M et al (2012) Iron repletion relocalizes hephaestin to a proximal basolateral compartment in polarized MDCK and Caco2 cells. Biochem Biophys Res Commun 421(3):449–455
Qian Z-M et al (2007) Development and iron-dependent expression of hephaestin in different brain regions of rats. J Cell Biochem 102(5):1225–1233
Wang J, Jiang H, Xie J-X (2007) Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. Eur J Neurosci 25(9):2766–2772
Cui R et al (2009) Age-dependent expression of hephaestin in the brain of ceruloplasmin-deficient mice. J Trace Elem Med Biol 23(4):290–299
Gitlin JD (1998) Aceruloplasminemia. Pediatr Res 44(3):271–276
Klomp LW et al (1996) Ceruloplasmin gene expression in the murine central nervous system. J Clin Invest 98(1):207–215
Chang YZ et al (2005) Effects of development and iron status on ceruloplasmin expression in rat brain. J Cell Physiol 204(2):623–631
Klomp LWJ, Gitlin JD (1996) Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. Hum Mol Genet 5(12):1989–1996
Patel BN, David S (1997) A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem 272(32):20185–20190
Patel BN, Dunn RJ, David S (2000) Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem 275(6):4305–4310
Mukhopadhyay CK, Attieh ZK, Fox PL (1998) Role of ceruloplasmin in cellular iron uptake. Science 279(5351):714–717
Attieh ZK et al (1999) Ceruloplasmin ferroxidase activity stimulates cellular iron uptake by a trivalent cation-specific transport mechanism. J Biol Chem 274(2):1116–1123
Gaasch J et al (2007) Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res 32(7):1196–1208
Marksteiner J, Humpel C (2007) Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol Psychiatry 13(10):939–952
Siman R et al (1989) Expression of 2-amyloid precursor protein in reactive astrocytes following neuronal damage. Neuron 3(3):275–285
Ford GC et al (1984) Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci 304(1121):551–565
Theil EC (1987) Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem 56(1):289–315
Aisen P, Listowsky I (1980) Iron transport and storage proteins. Annu Rev Biochem 49(1):357–393
Arosio P, Adelman TG, Drysdale JW (1978) On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem 253(12):4451–4458
Miller LL et al (1991) Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc Natl Acad Sci 88(11):4946–4950
Lawson DM et al (1989) Identification of the ferroxidase centre in ferritin. FEBS Lett 254(1–2):207–210
Levi S et al (1992) Evidence of H- and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem J 288(2):591–596
Rouault T, Zhang D-L, Jeong S (2009) Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 24(4):673–684
Li Z, Chen-Roetling J, Regan RF (2009) Increasing expression of H- or L-ferritin protects cortical astrocytes from hemin toxicity. Free Radic Res 43(6):613–621
Todorich B, Zhang X, Connor JR (2011) H-ferritin is the major source of iron for oligodendrocytes. Glia 59(6):927–935
Jacobson S (1963) Sequence of myelinization in the brain of the albino rat. A. Cerebral cortex, thalamus and related structures. J Comp Neurol 121(1):5–29
Kitazume S et al (2010) Brain endothelial cells produce amyloid β from amyloid precursor protein 770 and preferentially secrete the o-glycosylated form. J Biol Chem 285(51):40097–40103
Duce JA et al (2010) Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142(6):857–867
Ebrahimi KH, Hagedoorn P-L, Hagen WR (2012) A synthetic peptide with the putative iron binding motif of amyloid precursor protein (APP) does not catalytically oxidize iron. PLoS ONE 7(8):e40287
Honarmand Ebrahimi K et al (2013) The amyloid precursor protein (APP) does not have a ferroxidase site in its E2 domain. PLoS ONE 8(8):72177
McCarthy RC, Park YH, Kosman DJ (2014) sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep 15(7):809–815
Rogers JT et al (2008) Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer’s disease. Biochem Soc Trans 36(Pt 6):1282–1287
Cho HH et al (2010) Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem 285(41):31217–31232
Moos T (1995) Developmental profile of non-heme iron distribution in the rat brain during ontogenesis. Dev Brain Res 87(2):203–213
Hadziahmetovic M et al (2011) Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci 52(1):109–118
Wang SM et al (2010) Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci 67(1):123–133
Pellerin L, Magistretti PJ (2003) Food for thought: challenging the dogmas. J Cereb Blood Flow Metab 23:1282–1286
Newman EA (2003) New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci 26(10):536–542
Pelizzoni I et al (2013) Iron uptake in quiescent and inflammation-activated astrocytes: a potentially neuroprotective control of iron burden. Biochim Biophys Acta 1832(8):1326–1333
Zonta M et al (2003) Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50
Harris ZL et al (1995) Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci USA 92(7):2539–2543
Yoshida K et al (1995) A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nat Genet 9(3):267–272
Kaneko K et al (2002) Astrocytic deformity and globular structures are characteristic of the brains of patients with aceruloplasminemia. J Neuropathol Exp Neurol 61(12):1069–1077
Langer F et al (2011) Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J Neurosci 31(41):14488–14495
Tabaton M, Tamagno E (2007) The molecular link between β- and γ-secretase activity on the amyloid β precursor protein. Cell Mol Life Sci 64(17):2211–2218
Cai H et al (2001) BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci 4:233–234
Zheng H, Koo E (2006) The amyloid precursor protein: beyond amyloid. Mol Neurodegener 1(1):5
Bolognin S et al (2011) Aluminum, copper, iron and zinc differentially alter amyloid-Aβ1–42 aggregation and toxicity. Int J Biochem Cell Biol 43(6):877–885
Kayed R et al (2007) Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2(1):18
Broersen K, Rousseau F, Schymkowitz J (2010) The culprit behind amyloid beta peptide related neurotoxicity in Alzheimer’s disease: oligomer size or conformation? Alzheimers Res Ther 2(4):12
Everett J et al (2014) Ferrous iron formation following the co-aggregation of ferric iron and the Alzheimer’s disease peptide β-amyloid (1–42). J R Soc Interface 11(95)
Rottkamp CA et al (2001) Redox-active iron mediates amyloid-β toxicity. Free Radic Biol Med 30(4):447–450
Guo C et al (2012) Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 34:562–575
Guo C et al (2013) Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int 62(2):165–172
Aldred AR et al (1987) Distribution of transferrin synthesis in brain and other tissues in the rat. J Biol Chem 262(11):5293–5297
Giometto B et al (1990) Transferrin receptors in rat central nervous system: an immunocytochemical study. J Neurol Sci 98(1):81–90
Du F et al (2011) Hepcidin directly inhibits transferrin receptor 1 expression in astrocytes via a cyclic AMP-protein kinase a pathway. Glia 59(6):936–945
Espinosa de los Monteros A, Foucaud B (1987) Effect of iron and transferrin on pure oligodendrocytes in culture; characterization of a high-affinity transferrin receptor at different ages. Dev Brain Res 35(1):123–130
Moos T (1995) Increased accumulation of transferrin by motor neurons of the mouse mutant progressive motor neuronopathy (pmn/pmn). J Neurocytol 24(5):389–398
Moos T (1995) Age-dependent uptake and retrograde axonal transport of exogenous albumin and transferrin in rat motor neurons. Brain Res 672(1–2):14–23
He L et al (2006) ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharm 70(1):171–180
Iyengar V, Pullakhandam R, Nair KM (2009) Iron-zinc interaction during uptake in human intestinal Caco-2 cell line: Kinetic analyses and possible mechanism. Indian J Biochem Biophys 46:8
Liu Z et al (2008) Cd2+ versus Zn2+ uptake by the ZIP8-dependent symporter: kinetics, electrogenicity and trafficking. Biochem Biophys Res Commun 365(4):814–820
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22(23):3099–3108
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCarthy, R.C., Kosman, D.J. Iron transport across the blood–brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell. Mol. Life Sci. 72, 709–727 (2015). https://doi.org/10.1007/s00018-014-1771-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1771-4