Abstract
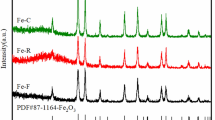
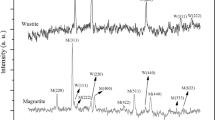
Two series of Fe/Cu/K catalysts were prepared by co-precipitation (CP-xK) and by co-precipitation accompanied with hydrothermal treatment (HY-xK), respectively. The catalysts were investigated by N2 adsorption, X-ray diffraction (XRD), Mössbauer effect spectroscopy (MES), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), H2 temperature-programmed reduction (H2-TPR) and thermogravimetric analysis. N2 adsorption revealed that HY-xK catalysts displayed relatively low surface area, but generated additional macropore. XRD and SEM showed that HY-xK catalysts were well grown crystallite, while the CP-xK catalysts were amorphous. In addition, hydrothermal treatment remarkably influenced the growth orientation of hematite nanocrystals, resulting in the preferential exposure of the (110) plane. HRTEM also indicated that HY-1.32K was dominated by Fe2O3 nanocrystals with (110) plane, and the CP-1.38K primary particle mainly exposed (104) plane. The H2-TPR profiles for the two series of catalysts were similar, though the merged and smooth peak of HY-xK catalysts possibly suggested that the component elements were more uniform. MES and XRD indicated that the catalyst after hydrothermal treatment can be easily reduced into active carbide phases in Fischer–Tropsch synthesis (FTS) reaction. In FTS reaction, the HY-xK catalysts showed higher activity and better stability than the CP-xK catalysts given comparable K content.
Graphical Abstract











Similar content being viewed by others
References
Bukur DB, Lang X (1999) Ind Eng Chem Res 38:3270
Dry ME (1990) Catal Lett 7:241
Dry ME (2002) Catal Today 71:227
Zhang CH, Yang Y, Teng BT, Li TZ, Zheng HY, Xiang HW, Li YW (2006) J Catal 237:405
O’Brien RJ, Davis BH (2004) Catal Lett 94:1
Dry ME, Shingles T, Boshoff LJ, Oosthuizen GJ (1969) J Catal 15:190
Arakawa H, Bell AT (1983) Ind Eng Chem Process Des Dev 22:97
Bukur DB, Mukesh D, Patel SA (1990) Ind Eng Chem Res 29:194
Dry ME, Oosthuizen G (1968) J Catal 11:18
Miller DG, Moskovits M (1988) J Phys Chem 92:6081
O’Brien RJ, Xu L, Spicer RL, Davis BH (1996) Energy Fuels 10:921
Benziger J, Madix R (1980) Surf Sci 94:119
Li S, Li A, Krishnamoorthy S, Iglesia E (2001) Catal Lett 77:197
Yang Y, Xiang HW, Xu YY, Bai L, Li YW (2004) Appl Catal A 266:181
Bukur DB, Lang X, Mukesh D, Zimmerman WH, Rosynek MP, Li C (1990) Ind Eng Chem Res 29:1588
Davis BH, Tungate FL (1991) DOE, Liquefaction contractors meeting, p 275
Shultz JF, Hofer LJE, Karn FS, Anderson RB (1962) J Phys Chem 66:501
Liu KK, Suo HY, Zhang C, Xu J, Yang Y, Xiang HW, Li YW (2010) Catal Commun 12:137
Schwuger M-J, Stickdorn K, Schomaecker R (1995) Chem Rev 95:849
Liu C, Zou B, Rondinone AJ, Zhang ZJ (2000) J Phys Chem B 104:1141
Calderone VR, Shiju NR, Curulla-Ferré D, Chambrey S, Khodakov A, Rose A, Thiessen J, Jess A, Rothenberg G (2013) Angew Chem Int Ed 52:4275
Dong HH, Xie MJ, Xu J, Li MF, Peng LM, Guo XF, Ding WP (2011) Chem Commun 47:4019
Kölbel H, Ralek M (1980) Catal Rev Sci Eng 21:225
Boudart M, McDonald MA (1984) J Phys Chem 88:2185
Feng S, Xu R (2000) Chem. Res 34:239
Mohapatra SK, John SE, Banerjee S, Misra M (2009) Chem Mater 21:3048
Qu J, Yin YX, Wang YQ, Yan Y, Guo YG, Song WG (2013) ACS Appl Mater Interfaces 5:3932
Sun JQ, Zheng SK, Zhang K, Song DC, Liu YT, Sun XD, Chen JG (2014) J Mater Chem 2:13116
Bukur DB, Koranne M, Lang X, Rao KRPM, Huffman GP (1995) Appl Catal A 126:85
Lox ES, Froment GF (1993) Ind Eng Chem Res 32:71
Huang CS, Xu L, Davis BH (1993) Fuel Sci Technol Int 11:639
Shroff MD, Kalakkad DS, Coulter KE, Kohler SD, Harrington MS, Jackson NB, Sault AG, Datye AK (1995) J Catal 156:185
Sirimanothan N, Hamdeh HH, Zhang Y, Davis BH (2002) Catal Lett 82:181
Ning WS, Koizumi N, Chang H, Mochizuki T, Itoh T, Yamada M (2006) Appl Catal A 312:35
Cui XJ, Xu J, Zhang CH, Yang Y, Gao P, Wu BS, Li YW (2011) J Catal 282:35
Cornell RM, Schwertmann U (1996) The iron oxides: structure, properties, reactions, occurences and uses. Second edition. VCH, Weinheim
Liu Z, Lv BL, Wu D, Sun YH, Xu Y (2012) Eur J Inorg Chem 2012:4076
Suo HY, Wang SG, Zhang CH, Xu J, Wu BS, Yang Y, Xiang HW, Li YW (2012) J Catal 286:111
Lohitharn N, Goodwin JG Jr (2008) J Catal 260:7
Rankin JL, Bartholomew CH (1986) J Catal 100:533
Kavitha MK, John H, Gopinath P (2014) Mater Res Bull 49:132
Mahajan D, Gütlich P, Stumm U (2003) Catal Commun 4:101
Herranz T, Rojas S, Perez-Alonso F, Ojeda M, Terreros P, Fierro J (2006) J Appl Catal A 311:66
Acknowledgments
The authors are indebted to the support from the National Natural Science Foundation of China (No. 21373254). This work is also supported by Wuhan Kaidi General Research Institute of Engineering & Technology Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, C., Chen, J. Effect of Hydrothermal Treatment on Precipitated Iron Catalyst for Fischer–Tropsch Synthesis. Catal Lett 145, 702–711 (2015). https://doi.org/10.1007/s10562-014-1457-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1457-4