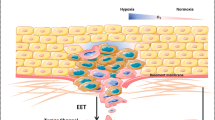
Abstract
Unexpected resistance to anti-angiogenic treatment prompted the investigation of non-angiogenic tumor processes. Vessel co-option (VC) and vasculogenic mimicry (VM) are recognized as primary non-angiogenic mechanisms. In VC, cancer cells utilize pre-existing blood vessels for support, whereas in VM, cancer cells channel and provide blood flow to rapidly growing tumors. Both processes have been implicated in the development of tumor and resistance to anti-angiogenic drugs in many tumor types. The morphology, but rare molecular alterations have been investigated in VC and VM. There is a pressing need to better understand the underlying cellular and molecular mechanisms. Here, we review the emerging circular RNA (circRNA)–mediated regulation of non-angiogenic processes, VC and VM.



Similar content being viewed by others
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- ARP2/3:
-
Actin-related protein 2/3
- BEV:
-
Bevacizumab
- CAMs:
-
Cell adhesion molecules
- CC:
-
Cervical cancer
- CCRCC:
-
Clear cell renal cell carcinoma
- circRNA:
-
Circular RNA
- CRC:
-
Colorectal cancer
- CSC:
-
Cancer stem cells
- CXCL12:
-
CXC motif chemokine ligand 12
- CXCR4:
-
CXC motif chemokine receptor 4
- ECM:
-
Extracellular matrix
- EBV:
-
Epstein-Barr virus
- EMT:
-
Epithelial-to-mesenchymal transition
- EphA2:
-
Erythropoietin-producing hepatocellular receptor A2
- ER:
-
Endoplasmic reticulum
- EVs:
-
Endothelium-dependent vessels
- ERK1/2:
-
Extracellular signal-regulated kinase 1 and 2
- EVMM:
-
Extravascular migratory metastasis
- FAK:
-
Focal adhesion kinase
- GBM:
-
Glioblastoma
- GC:
-
Gastric cancer
- HCC:
-
Hepatocellular carcinoma
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HUVECs:
-
Human umbilical vein endothelial cells
- IL-8:
-
Interleukin-8
- IRE1:
-
Inositol-requiring enzyme 1
- JMY:
-
Junctional mediating and regulator Y
- L1CAM:
-
L1 cell adhesion molecule
- MDM2:
-
Mouse double minute 2
- miRNA:
-
MicroRNA
- MMP2:
-
Matrix metalloproteinase 2
- MV:
-
Mosaic vessels
- NPC:
-
Nasopharyngeal carcinoma
- NSCLC:
-
Non-small cell lung cancer
- OC:
-
Ovarian cancer
- OS:
-
Overall survival
- RCC:
-
Renal cell carcinoma
- SNAIL2:
-
Transcription factor Slug
- THBS1:
-
Thrombospondin-1
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple-negative breast cancer
- VC:
-
Vessel co-option
- VE:
-
Vascular endothelial
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
VEGF receptors
- VM:
-
Vasculogenic mimicry
References
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100(1), 57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Vasudev, N. S., & Reynolds, A. R. (2014). Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. [; Research Support, Non-U.S. Gov’t Review]. Angiogenesis, 17(3), 471–494. https://doi.org/10.1007/s10456-014-9420-y
Jayson, G. C., Kerbel, R., Ellis, L. M., & Harris, A. L. (2016). Antiangiogenic therapy in oncology: Current status and future directions. Lancet, 388(10043), 518–529. https://doi.org/10.1016/s0140-6736(15)01088-0
Cloughesy, T. F., Brenner, A., de Groot, J. F., Butowski, N. A., Zach, L., Campian, J. L., et al. (2020). A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro-Oncology, 22(5), 705–717. https://doi.org/10.1093/neuonc/noz232
Zeng, Y., & Fu, B. M. (2020). Resistance mechanisms of anti-angiogenic therapy and exosomes-mediated revascularization in cancer. Frontiers in Cell and Developmental Biology, 8, 610661. https://doi.org/10.3389/fcell.2020.610661
Kuczynski, E. A., Vermeulen, P. B., Pezzella, F., Kerbel, R. S., & Reynolds, A. R. (2019). Vessel co-option in cancer. Nature Reviews Clinical Oncology, 16(8), 469–493. https://doi.org/10.1038/s41571-019-0181-9
Zhang, J., Qiao, L., Liang, N., Xie, J., Luo, H., Deng, G., et al. (2016). Vasculogenic mimicry and tumor metastasis. Journal of B.U.ON., 21(3), 533–541.
Latacz, E., Caspani, E., Barnhill, R., Lugassy, C., Verhoef, C., Grunhagen, D., et al. (2020). Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis, 23(1), 43–54. https://doi.org/10.1007/s10456-019-09690-0
Kuczynski, E. A., & Reynolds, A. R. (2020). Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis, 23(1), 55–74. https://doi.org/10.1007/s10456-019-09698-6
Fathi Maroufi, N., Taefehshokr, S., Rashidi, M.-R., Taefehshokr, N., Khoshakhlagh, M., Isazadeh, A., et al. (2020). Vascular mimicry: Changing the therapeutic paradigms in cancer. Molecular Biology Reports, 47(6), 4749–4765. https://doi.org/10.1007/s11033-020-05515-2
Kristensen, L. S., Andersen, M. S., Stagsted, L. V. W., Ebbesen, K. K., Hansen, T. B., & Kjems, J. (2019). The biogenesis, biology and characterization of circular RNAs. Nature Reviews Genetics, 20(11), 675–691. https://doi.org/10.1038/s41576-019-0158-7
Shao, Y., Lu, B. (2020). The crosstalk between circular RNAs and the tumor microenvironment in cancer metastasis. Cancer Cell International, 20(1), https://doi.org/10.1186/s12935-020-01532-0
Pezzella, F., Di Bacco, A., Andreola, S., Nicholson, A. G., Pastorino, U., & Harris, A. L. (1996). Angiogenesis in primary lung cancer and lung secondaries. The European Journal of Cancer, 32a(14), 2494–2500. https://doi.org/10.1016/s0959-8049(96)00377-2
Pezzella, F., Pastorino, U., Tagliabue, E., Andreola, S., Sozzi, G., Gasparini, G., et al. (1997). Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. American Journal of Pathology, 151(5), 1417–1423.
Lugassy, C., Zadran, S., Bentolila, L. A., Wadehra, M., Prakash, R., Carmichael, S. T., et al. (2014). Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: An alternative to intravascular cancer dissemination. Cancer Microenvironment, 7(3), 139–152. https://doi.org/10.1007/s12307-014-0156-4
Baker, G. J., Yadav, V. N., Motsch, S., Koschmann, C., Calinescu, A. A., Mineharu, Y., et al. (2014). Mechanisms of glioma formation: Iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia, 16(7), 543–561. https://doi.org/10.1016/j.neo.2014.06.003
Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia (2009). Hepatology, 49(2), 658–664, https://doi.org/10.1002/hep.22709
van Dam, P. J., van der Stok, E. P., Teuwen, L. A., Van den Eynden, G. G., Illemann, M., Frentzas, S., et al. (2017). International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. British Journal of Cancer, 117(10), 1427–1441. https://doi.org/10.1038/bjc.2017.334
Rada, M., Lazaris, A., Kapelanski-Lamoureux, A., Mayer, T. Z., & Metrakos, P. (2020). Tumor microenvironment conditions that favor vessel co-option in colorectal cancer liver metastases: A theoretical model. Seminars in Cancer Biology. https://doi.org/10.1016/j.semcancer.2020.09.001
Griveau, A., Seano, G., Shelton, S. J., Kupp, R., Jahangiri, A., Obernier, K., et al. (2018). A glial signature and wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell, 33(5), 874-889.e877. https://doi.org/10.1016/j.ccell.2018.03.020
Kuczynski, E. A., Yin, M., Bar-Zion, A., Lee, C. R., Butz, H., Man, S., et al. (2016). Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. Journal of the National Cancer Institute, 108(8), https://doi.org/10.1093/jnci/djw030
Voutouri, C., Kirkpatrick, N. D., Chung, E., Mpekris, F., Baish, J. W., Munn, L. L., et al. (2019). Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proceedings of the National Academy of Sciences of the United States of America, 116(7), 2662–2671. https://doi.org/10.1073/pnas.1818322116
Bridgeman, V. L., Vermeulen, P. B., Foo, S., Bilecz, A., Daley, F., Kostaras, E., et al. (2017). Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. Journal of Pathology, 241(3), 362–374. https://doi.org/10.1002/path.4845
Dome, B., Timar, J., & Paku, S. (2003). A novel concept of glomeruloid body formation in experimental cerebral metastases. Journal of Neuropathology and Experimental Neurology, 62(6), 655–661.
Stessels, F., Van den Eynden, G., Van der Auwera, I., Salgado, R., Van den Heuvel, E., Harris, A. L., et al. (2004). Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. British Journal of Cancer, 90(7), 1429–1436. https://doi.org/10.1038/sj.bjc.6601727
Vermeulen, P. B., Colpaert, C., Salgado, R., Royers, R., Hellemans, H., Van den Heuvel, E., et al. (2001). Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. Journal of Pathology, 195(3), 336–342. https://doi.org/10.1002/path.966
Kita, K., Itoshima, T., & Tsuji, T. (1991). Observation of microvascular casts of human hepatocellular-carcinoma by scanning electron-microscopy. Gastroenterologia Japonica, 26(3), 319–328.
Ebos, J. M., & Kerbel, R. S. (2011). Antiangiogenic therapy: Impact on invasion, disease progression, and metastasis. Nature Reviews. Clinical Oncology, 8(4), 210–221. https://doi.org/10.1038/nrclinonc.2011.21
Roviello, G., Bachelot, T., Hudis, C. A., Curigliano, G., Reynolds, A. R., Petrioli, R., et al. (2017). The role of bevacizumab in solid tumours: A literature based meta-analysis of randomised trials. European Journal of Cancer, 75, 245–258. https://doi.org/10.1016/j.ejca.2017.01.026
Porta, C., Cosmai, L., Leibovich, B. C., Powles, T., Gallieni, M., & Bex, A. (2019). The adjuvant treatment of kidney cancer: A multidisciplinary outlook. Nature reviews. Nephrology, 15(7), 423–433. https://doi.org/10.1038/s41581-019-0131-x
Frentzas, S., Simoneau, E., Bridgeman, V. L., Vermeulen, P. B., Foo, S., Kostaras, E., et al. (2016). Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nature Medicine, 22(11), 1294–1302. https://doi.org/10.1038/nm.4197
Kölsch, V., Charest, P. G., & Firtel, R. A. (2008). The regulation of cell motility and chemotaxis by phospholipid signaling. Journal of Cell Science, 121(Pt 5), 551–559. https://doi.org/10.1242/jcs.023333
Lugassy, C., & Barnhill, R. L. (2007). Angiotropic melanoma and extravascular migratory metastasis: A review. Advances in Anatomic Pathology, 14(3), 195–201. https://doi.org/10.1097/PAP.0b013e31805048d9
Watkins, S., Robel, S., Kimbrough, I. F., Robert, S. M., Ellis-Davies, G., & Sontheimer, H. (2014). Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nature Communications, 5, 4196. https://doi.org/10.1038/ncomms5196
Kienast, Y., von Baumgarten, L., Fuhrmann, M., Klinkert, W. E., Goldbrunner, R., Herms, J., et al. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nature Medicine, 16(1), 116–122. https://doi.org/10.1038/nm.2072
Garcia-Gomez, P., & Valiente, M. (2020). Vascular co-option in brain metastasis. Angiogenesis, 23(1), 3–8. https://doi.org/10.1007/s10456-019-09693-x
Caspani, E. M., Crossley, P. H., Redondo-Garcia, C., & Martinez, S. (2014). Glioblastoma: A pathogenic crosstalk between tumor cells and pericytes. PLoS ONE, 9(7), e101402. https://doi.org/10.1371/journal.pone.0101402
Tse, J. M., Cheng, G., Tyrrell, J. A., Wilcox-Adelman, S. A., Boucher, Y., Jain, R. K., et al. (2012). Mechanical compression drives cancer cells toward invasive phenotype. Proceedings of the National Academy of Sciences of the United States of America, 109(3), 911–916. https://doi.org/10.1073/pnas.1118910109
Butler, J. M., Kobayashi, H., & Rafii, S. (2010). Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nature Reviews Cancer, 10(2), 138–146. https://doi.org/10.1038/nrc2791
Sharma, I., Singh, A., Siraj, F., & Saxena, S. (2018). IL-8/CXCR1/2 signalling promotes tumor cell proliferation, invasion and vascular mimicry in glioblastoma. Journal of Biomedical Science, 25(1), 62. https://doi.org/10.1186/s12929-018-0464-y
Seano, G., & Jain, R. K. (2020). Vessel co-option in glioblastoma: Emerging insights and opportunities. Angiogenesis, 23(1), 9–16. https://doi.org/10.1007/s10456-019-09691-z
Pham, K., Luo, D., Siemann, D. W., Law, B. K., Reynolds, B. A., Hothi, P., et al. (2015). VEGFR inhibitors upregulate CXCR4 in VEGF receptor-expressing glioblastoma in a TGFβR signaling-dependent manner. Cancer Letters, 360(1), 60–67. https://doi.org/10.1016/j.canlet.2015.02.005
Donnem, T., Reynolds, A. R., Kuczynski, E. A., Gatter, K., Vermeulen, P. B., Kerbel, R. S., et al. (2018). Non-angiogenic tumours and their influence on cancer biology. Nature Reviews Cancer, 18(5), 323–336. https://doi.org/10.1038/nrc.2018.14
Palmieri, V., Lazaris, A., Mayer, T. Z., Petrillo, S. K., Alamri, H., Rada, M., et al. (2020). Neutrophils expressing lysyl oxidase-like 4 protein are present in colorectal cancer liver metastases resistant to anti-angiogenic therapy. The Journal of Pathology, 251(2), 213–223. https://doi.org/10.1002/path.5449
Sun, D., Liu, J., & Zhou, L. (2019). Upregulation of circular RNA circ-FAM53B predicts adverse prognosis and accelerates the progression of ovarian cancer via the miR-646/VAMP2 and miR-647/MDM2 signaling pathways. Oncology Reports, 42(6), 2728–2737. https://doi.org/10.3892/or.2019.7366
Zhang, F., Xu, Y., Ye, W., Jiang, J., & Wu, C. (2020). Circular RNA S-7 promotes ovarian cancer EMT via sponging miR-641 to up-regulate ZEB1 and MDM2. Bioscience Reports, 40(7), https://doi.org/10.1042/BSR20200825
Yanbin, Z., & Jing, Z. (2019). CircSAMD4A accelerates cell proliferation of osteosarcoma by sponging miR-1244 and regulating MDM2 mRNA expression. Biochemical and Biophysical Research Communications, 516(1), 102–111. https://doi.org/10.1016/j.bbrc.2019.05.182
Jin, Y., Li, L., Zhu, T., & Liu, G. (2019). Circular RNA circ_0102049 promotes cell progression as ceRNA to target MDM2 via sponging miR-1304-5p in osteosarcoma. Pathology - Research and Practice, 215(12), 152688. https://doi.org/10.1016/j.prp.2019.152688
Ma, W., Zhao, P., Zang, L., Zhang, K., Liao, H., & Hu, Z. (2020). CircTP53 promotes the proliferation of thyroid cancer via targeting miR-1233-3p/MDM2 axis. Journal of Endocrinological Investigation. https://doi.org/10.1007/s40618-020-01317-2
Liu, R., Zhou, M., Zhang, P., Zhao, Y., & Zhang, Y. (2020). Cell proliferation and invasion is promoted by circSERPINA3 in nasopharyngeal carcinoma by regulating miR-944/MDM2 axis. Journal of Cancer, 11(13), 3910–3918. https://doi.org/10.7150/jca.42799
Wang, K., Sun, Y., Tao, W., Fei, X., & Chang, C. (2017). Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Letters, 394, 1–12. https://doi.org/10.1016/j.canlet.2016.12.036
Xing, L., Yang, R., Wang, X., Zheng, X., Yang, X., Zhang, L., et al. (2020). The circRNA circIFI30 promotes progression of triple-negative breast cancer and correlates with prognosis. Aging (Albany NY), 12(11), 10983–11003. https://doi.org/10.18632/aging.103311
Hong, Y., Qin, H., Li, Y., Zhang, Y., Zhuang, X., Liu, L., et al. (2019). FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. Journal of Cellular Physiology, 234(11), 19895–19910. https://doi.org/10.1002/jcp.28588
Ma, H. B., Yao, Y. N., Yu, J. J., Chen, X. X., & Li, H. F. (2018). Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. American Journal of Translational Research, 10(2), 592–604.
Du, W. W., Yang, W., Li, X., Fang, L., Wu, N., Li, F., et al. (2020). The circular RNA circSKA3 binds integrin beta1 to induce invadopodium formation enhancing breast cancer invasion. Molecular Therapy, 28(5), 1287–1298. https://doi.org/10.1016/j.ymthe.2020.03.002
Yang, W., & Xie, T. (2020). Hsa_circ_CSPP1/MiR-361-5p/ITGB1 regulates proliferation and migration of cervical cancer (CC) by modulating the PI3K-Akt signaling pathway. Reproductive Sciences, 27(1), 132–144. https://doi.org/10.1007/s43032-019-00008-5
Wang, N., Lu, K., Qu, H., Wang, H., Chen, Y., Shan, T., et al. (2020). CircRBM33 regulates IL-6 to promote gastric cancer progression through targeting miR-149. Biomedicine & Pharmacotherapy, 125, 109876. https://doi.org/10.1016/j.biopha.2020.109876
Xu, Z., Tie, X., Li, N., Yi, Z., Shen, F., & Zhang, Y. (2020). Circular RNA hsa_circ_0000654 promotes esophageal squamous cell carcinoma progression by regulating the miR-149-5p/IL-6/STAT3 pathway. IUBMB Life, 72(3), 426–439. https://doi.org/10.1002/iub.2202
Zhang, P. F., Pei, X., Li, K. S., Jin, L. N., Wang, F., Wu, J., et al. (2019). Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Molecular Cancer, 18(1), 179. https://doi.org/10.1186/s12943-019-1111-2
Li, H., Yao, G., Feng, B., Lu, X., & Fan, Y. (2018). Circ_0056618 and CXCR4 act as competing endogenous in gastric cancer by regulating miR-206. Journal of Cellular Biochemistry, 119(11), 9543–9551. https://doi.org/10.1002/jcb.27271
Zheng, X., Ma, Y. F., Zhang, X. R., Li, Y., Zhao, H. H., & Han, S. G. (2020). Circ_0056618 promoted cell proliferation, migration and angiogenesis through sponging with miR-206 and upregulating CXCR4 and VEGF-A in colorectal cancer. European Review for Medical and Pharmacological Sciences, 24(8), 4190–4202. https://doi.org/10.26355/eurrev_202004_20999
He, F., Zhong, X., Lin, Z., Lin, J., Qiu, M., Li, X., et al. (2020). Plasma exo-hsa_circRNA_0056616: A potential biomarker for lymph node metastasis in lung adenocarcinoma. Journal of Cancer, 11(14), 4037–4046. https://doi.org/10.7150/jca.30360
Adighibe, O., & Pezzella, F. (2018). The Role of JMY in p53 Regulation. Cancers (Basel), 10(6), https://doi.org/10.3390/cancers10060173
Coutts, A. S., Weston, L., & La Thangue, N. B. (2010). Actin nucleation by a transcription co-factor that links cytoskeletal events with the p53 response. Cell Cycle, 9(8), 1511–1515. https://doi.org/10.4161/cc.9.8.11258
Coutts, A. S., Boulahbel, H., Graham, A., & La Thangue, N. B. (2007). Mdm2 targets the p53 transcription cofactor JMY for degradation. EMBO Reports, 8(1), 84–90. https://doi.org/10.1038/sj.embor.7400855
Talme, T., Bergdahl, E., & Sundqvist, K. G. (2014). Regulation of T-lymphocyte motility, adhesion and de-adhesion by a cell surface mechanism directed by low density lipoprotein receptor-related protein 1 and endogenous thrombospondin-1. Immunology, 142(2), 176–192. https://doi.org/10.1111/imm.12229
Daubon, T., Léon, C., Clarke, K., Andrique, L., Salabert, L., Darbo, E., et al. (2019). Deciphering the complex role of thrombospondin-1 in glioblastoma development. Nature Communications, 10(1), 1146. https://doi.org/10.1038/s41467-019-08480-y
Skandalis, S. S., Karalis, T. T., Chatzopoulos, A., & Karamanos, N. K. (2019). Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cellular Signalling, 63, 109377. https://doi.org/10.1016/j.cellsig.2019.109377
Lazaris, A., Amri, A., Petrillo, S. K., Zoroquiain, P., Ibrahim, N., Salman, A., et al. (2018). Vascularization of colorectal carcinoma liver metastasis: Insight into stratification of patients for anti-angiogenic therapies. The Journal of Pathology: Clinical Research, 4(3), 184–192. https://doi.org/10.1002/cjp2.100
Samatov, T. R., Wicklein, D., & Tonevitsky, A. G. (2016). L1CAM: Cell adhesion and more. Progress in Histochemistry and Cytochemistry, 51(2), 25–32. https://doi.org/10.1016/j.proghi.2016.05.001
Valiente, M., Obenauf, A. C., Jin, X., Chen, Q., Zhang, X. H., Lee, D. J., et al. (2014). Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 156(5), 1002–1016. https://doi.org/10.1016/j.cell.2014.01.040
Er, E. E., Valiente, M., Ganesh, K., Zou, Y., Agrawal, S., Hu, J., et al. (2018). Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nature Cell Biology, 20(8), 966–978. https://doi.org/10.1038/s41556-018-0138-8
Bugyik, E., Dezso, K., Reiniger, L., László, V., Tóvári, J., Tímár, J., et al. (2011). Lack of angiogenesis in experimental brain metastases. Journal of Neuropathology and Experimental Neurology, 70(11), 979–991. https://doi.org/10.1097/NEN.0b013e318233afd7
Yao, H., Price, T. T., Cantelli, G., Ngo, B., Warner, M. J., Olivere, L., et al. (2018). Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature, 560(7716), 55–60. https://doi.org/10.1038/s41586-018-0342-5
Carbonell, W. S., DeLay, M., Jahangiri, A., Park, C. C., & Aghi, M. K. (2013). β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Research, 73(10), 3145–3154. https://doi.org/10.1158/0008-5472.can-13-0011
Chen, D. L., Zeng, Z. L., Yang, J., Ren, C., Wang, D. S., Wu, W. J., et al. (2013). L1cam promotes tumor progression and metastasis and is an independent unfavorable prognostic factor in gastric cancer. Journal of Hematology & Oncology, 6, 43. https://doi.org/10.1186/1756-8722-6-43
Colombo, F., & Meldolesi, J. (2015). L1-CAM and N-CAM: From adhesion proteins to pharmacological targets. Trends in Pharmacological Sciences, 36(11), 769–781. https://doi.org/10.1016/j.tips.2015.08.004
Carbonell, W. S., Ansorge, O., Sibson, N., & Muschel, R. (2009). The vascular basement membrane as “soil” in brain metastasis. PLoS ONE, 4(6), e5857. https://doi.org/10.1371/journal.pone.0005857
Eddy, R. J., Weidmann, M. D., Sharma, V. P., & Condeelis, J. S. (2017). Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends in Cell Biology, 27(8), 595–607. https://doi.org/10.1016/j.tcb.2017.03.003
Lorenz, M., Yamaguchi, H., Wang, Y., Singer, R. H., & Condeelis, J. (2004). Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Current Biology, 14(8), 697–703. https://doi.org/10.1016/j.cub.2004.04.008
Marx, J. (2006). Cell biology. Podosomes and invadopodia help mobile cells step lively. Science, 312(5782), 1868–1869. https://doi.org/10.1126/science.312.5782.1868
Kumari, N., Dwarakanath, B. S., Das, A., & Bhatt, A. N. (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biology, 37(9), 11553–11572. https://doi.org/10.1007/s13277-016-5098-7
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741. https://doi.org/10.1158/1078-0432.ccr-07-4843
Auf, G., Jabouille, A., Guérit, S., Pineau, R., Delugin, M., Bouchecareilh, M., et al. (2010). Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15553–15558. https://doi.org/10.1073/pnas.0914072107
Ron, D., & Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology, 8(7), 519–529. https://doi.org/10.1038/nrm2199
Donnem, T., Hu, J., Ferguson, M., Adighibe, O., Snell, C., Harris, A. L., et al. (2013). Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Medicine, 2(4), 427–436. https://doi.org/10.1002/cam4.105
Ohe, K., Tanaka, T., Horita, Y., Harada, Y., Yamasaki, T., Abe, I., et al. (2019). Circular IRE-type RNAs of the NR5A1 gene are formed in adrenocortical cells. Biochemical and Biophysical Research Communications, 512(1), 1–6. https://doi.org/10.1016/j.bbrc.2019.02.151
Ruf, W., Seftor, E. A., Petrovan, R. J., Weiss, R. M., Gruman, L. M., Margaryan, N. V., et al. (2003). Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Research, 63(17), 5381–5389.
Wechman, S. L., Emdad, L., Sarkar, D., Das, S. K., & Fisher, P. B. (2020). Vascular mimicry: Triggers, molecular interactions and in vivo models. Advances in Cancer Research, 148, 27–67. https://doi.org/10.1016/bs.acr.2020.06.001.
Ge, H., & Luo, H. (2018). Overview of advances in vasculogenic mimicry - A potential target for tumor therapy. Cancer Management and Research, 10, 2429–2437. https://doi.org/10.2147/cmar.s164675
Karroum, A., Mirshahi, P., Benabbou, N., Faussat, A. M., Soria, J., Therwath, A., et al. (2010). Matrix metalloproteinase-9 is required for tubular network formation and migration of resistant breast cancer cells MCF-7 through PKC and ERK1/2 signalling pathways. Cancer Letters, 295(2), 242–251. https://doi.org/10.1016/j.canlet.2010.03.007
Lim, D., Do, Y., Kwon, B. S., Chang, W., Lee, M. S., Kim, J., et al. (2020). Angiogenesis and vasculogenic mimicry as therapeutic targets in ovarian cancer. BMB Reports, 53(6), 291–298. https://doi.org/10.5483/BMBRep.2020.53.6.060
Huang, B., Xiao, E., & Huang, M. (2015). MEK/ERK pathway is positively involved in hypoxia-induced vasculogenic mimicry formation in hepatocellular carcinoma which is regulated negatively by protein kinase A. Medical Oncology, 32(1), 408. https://doi.org/10.1007/s12032-014-0408-7
Williamson, S. C., Metcalf, R. L., Trapani, F., Mohan, S., Antonello, J., Abbott, B., et al. (2016). Vasculogenic mimicry in small cell lung cancer. Nature Communications, 7, 13322. https://doi.org/10.1038/ncomms13322
Xu, Y., Li, Q., Li, X. Y., Yang, Q. Y., Xu, W. W., & Liu, G. L. (2012). Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. Journal of Experimental & Clinical Cancer Research, 31(1), 16. https://doi.org/10.1186/1756-9966-31-16
Hu, Y. L., DeLay, M., Jahangiri, A., Molinaro, A. M., Rose, S. D., Carbonell, W. S., et al. (2012). Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Research, 72(7), 1773–1783. https://doi.org/10.1158/0008-5472.can-11-3831
Angara, K., Borin, T. F., Rashid, M. H., Lebedyeva, I., Ara, R., Lin, P. C., et al. (2018). CXCR2-expressing tumor cells drive vascular mimicry in antiangiogenic therapy-resistant glioblastoma. Neoplasia, 20(10), 1070–1082. https://doi.org/10.1016/j.neo.2018.08.011
Liu, R., Yang, K., Meng, C., Zhang, Z., & Xu, Y. (2012). Vasculogenic mimicry is a marker of poor prognosis in prostate cancer. Cancer Biology & Therapy, 13(7), 527–533. https://doi.org/10.4161/cbt.19602
Valdivia, A., Mingo, G., Aldana, V., Pinto, M. P., Ramirez, M., Retamal, C., et al. (2019). Fact or fiction, it is time for a verdict on vasculogenic mimicry? Frontiers in Oncology, 9, 680. https://doi.org/10.3389/fonc.2019.00680
Zhang, X., Zhang, J., Zhou, H., Fan, G., & Li, Q. (2019). Molecular mechanisms and anticancer therapeutic strategies in vasculogenic mimicry. Journal of Cancer, 10(25), 6327–6340. https://doi.org/10.7150/jca.34171
Sun, T., Zhao, N., Zhao, X. L., Gu, Q., Zhang, S. W., Che, N., et al. (2010). Expression and functional significance of Twist1 in hepatocellular carcinoma: Its role in vasculogenic mimicry. Hepatology, 51(2), 545–556. https://doi.org/10.1002/hep.23311
Gong, W., Sun, B., Zhao, X., Zhang, D., Sun, J., Liu, T., et al. (2016). Nodal signaling promotes vasculogenic mimicry formation in breast cancer via the Smad2/3 pathway. Oncotarget, 7(43), 70152–70167. https://doi.org/10.18632/oncotarget.12161
Bao, S., Jin, S., Wang, C., Tu, P., Hu, K., & Lu, J. (2020). Androgen receptor suppresses vasculogenic mimicry in hepatocellular carcinoma via circRNA7/miRNA7-5p/VE-cadherin/Notch4 signalling. Journal of Cellular and Molecular Medicine, 24(23), 14110–14120. https://doi.org/10.1111/jcmm.16022
Zan, S. J., Zhao, Y., Fang, T., & Chen, K. (2019). Expression of circular RNA hsa_circ_0014130 in lung adenocarcinoma cell lines and its effect on proliferation and invasion of lung adenocarcinoma cell line. Zhonghua Bing Li Xue Za Zhi, 48(12), 934–939. https://doi.org/10.3760/cma.j.issn.0529-5807.2019.12.004
Zhou, P., Xie, W., Huang, H. L., Huang, R. Q., Tian, C., Zhu, H. B., et al. (2020). circRNA_100859 functions as an oncogene in colon cancer by sponging the miR-217-HIF-1α pathway. Aging (Albany NY), 12(13), 13338–13353. https://doi.org/10.18632/aging.103438
Zhai, Z., Fu, Q., Liu, C., Zhang, X., Jia, P., Xia, P., et al. (2019). Emerging roles of hsa-circ-0046600 targeting the miR-640/HIF-1α signalling pathway in the progression of HCC. Oncotargets and Therapy, 12, 9291–9302. https://doi.org/10.2147/ott.s229514
Qian, W., Huang, T., & Feng, W. (2020). Circular RNA HIPK3 promotes EMT of cervical cancer through sponging miR-338-3p to up-regulate HIF-1α. Cancer Management and Research, 12, 177–187. https://doi.org/10.2147/cmar.s232235
Shangguan, H., Feng, H., Lv, D., Wang, J., Tian, T., & Wang, X. (2020). Circular RNA circSLC25A16 contributes to the glycolysis of non-small-cell lung cancer through epigenetic modification. Cell Death & Disease, 11(6), 437. https://doi.org/10.1038/s41419-020-2635-5
Xu, G., Li, M., Wu, J., Qin, C., Tao, Y., & He, H. (2020). Circular RNA circNRIP1 sponges microRNA-138-5p to maintain hypoxia-induced resistance to 5-fluorouracil through hif-1α-dependent glucose metabolism in gastric carcinoma. Cancer Management and Research, 12, 2789–2802. https://doi.org/10.2147/cmar.s246272
Peng, G., Meng, H., Pan, H., & Wang, W. (2021). CircRNA 001418 promoted cell growth and metastasis of bladder carcinoma via EphA2 by miR-1297. Current Molecular Pharmacology, 14(1), 68–78. https://doi.org/10.2174/1874467213666200505093815
Liu, L., Yang, X., Li, N. F., Lin, L., & Luo, H. (2019). Circ_0015756 promotes proliferation, invasion and migration by microRNA-7-dependent inhibition of FAK in hepatocellular carcinoma. Cell Cycle, 18(21), 2939–2953. https://doi.org/10.1080/15384101.2019.1664223
Wu, D., Xia, A., Fan, T., & Li, G. (2021). circRASGRF2 functions as an oncogenic gene in hepatocellular carcinoma by acting as a miR-1224 sponge. Molecular Therapy - Nucleic Acids, 23, 13–26. https://doi.org/10.1016/j.omtn.2020.10.035
Guan, X., Zong, Z. H., Liu, Y., Chen, S., Wang, L. L., & Zhao, Y. (2019). circPUM1 promotes tumorigenesis and progression of ovarian cancer by sponging miR-615-5p and miR-6753-5p. Molecular Therapy - Nucleic Acids, 18, 882–892. https://doi.org/10.1016/j.omtn.2019.09.032
He, J. H., Han, Z. P., Luo, J. G., Jiang, J. W., Zhou, J. B., Chen, W. M., et al. (2020). Hsa_Circ_0007843 Acts as a mIR-518c-5p sponge to regulate the migration and invasion of colon cancer SW480 Cells. Frontiers in Genetics, 11, 9. https://doi.org/10.3389/fgene.2020.00009
Liu, H., Hu, G., Wang, Z., Liu, Q., Zhang, J., Chen, Y., et al. (2020). circPTCH1 promotes invasion and metastasis in renal cell carcinoma via regulating miR-485-5p/MMP14 axis. Theranostics, 10(23), 10791–10807. https://doi.org/10.7150/thno.47239
Xiao, H., & Liu, M. (2020). Circular RNA hsa_circ_0053277 promotes the development of colorectal cancer by upregulating matrix metallopeptidase 14 via miR-2467-3p sequestration. Journal of Cellular Physiology, 235(3), 2881–2890. https://doi.org/10.1002/jcp.29193
Delgado-Bellido, D., Serrano-Saenz, S., Fernández-Cortés, M., & Oliver, F. J. (2017). Vasculogenic mimicry signaling revisited: Focus on non-vascular VE-cadherin. Molecular Cancer, 16(1), 65. https://doi.org/10.1186/s12943-017-0631-x
Seftor, E. A., Meltzer, P. S., Schatteman, G. C., Gruman, L. M., Hess, A. R., Kirschmann, D. A., et al. (2002). Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: Role in vasculogenic mimicry. Critical Reviews in Oncology Hematology, 44(1), 17–27. https://doi.org/10.1016/s1040-8428(01)00199-8
Bartolomé, R. A., Torres, S., Isern de Val, S., Escudero-Paniagua, B., Calviño, E., Teixidó, J., et al. (2017). VE-cadherin RGD motifs promote metastasis and constitute a potential therapeutic target in melanoma and breast cancers. Oncotarget, 8(1), 215–227. https://doi.org/10.18632/oncotarget.13832
Hendrix, M. J., Seftor, E. A., Hess, A. R., & Seftor, R. E. (2003). Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nature Reviews Cancer, 3(6), 411–421. https://doi.org/10.1038/nrc1092
Yang, J., Zhu, D. M., Zhou, X. G., Yin, N., Zhang, Y., Zhang, Z. X., et al. (2017). HIF-2α promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget, 8(29), 47801–47815. https://doi.org/10.18632/oncotarget.17999
McAllister, J. C., Zhan, Q., Weishaupt, C., Hsu, M. Y., & Murphy, G. F. (2010). The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. Journal of Cutaneous Pathology, 37 Suppl 1(Suppl 1), 19–25. https://doi.org/10.1111/j.1600-0560.2010.01503.x
Topczewska, J. M., Postovit, L. M., Margaryan, N. V., Sam, A., Hess, A. R., Wheaton, W. W., et al. (2006). Embryonic and tumorigenic pathways converge via Nodal signaling: Role in melanoma aggressiveness. Nature Medicine, 12(8), 925–932. https://doi.org/10.1038/nm1448
Han, H., Du, L., Cao, Z., Zhang, B., & Zhou, Q. (2018). Triptonide potently suppresses pancreatic cancer cell-mediated vasculogenic mimicry by inhibiting expression of VE-cadherin and chemokine ligand 2 genes. European Journal of Pharmacology, 818, 593–603. https://doi.org/10.1016/j.ejphar.2017.11.019
Cao, W., Xu, C., Li, X., & Yang, X. (2019). Twist1 promotes astrocytoma development by stimulating vasculogenic mimicry. Oncology Letters, 18(1), 846–855. https://doi.org/10.3892/ol.2019.10380
Mao, X. G., Xue, X. Y., Wang, L., Zhang, X., Yan, M., Tu, Y. Y., et al. (2013). CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia. Neuro-Oncology, 15(7), 865–879. https://doi.org/10.1093/neuonc/not029
Hess, A. R., Seftor, E. A., Gruman, L. M., Kinch, M. S., Seftor, R. E., & Hendrix, M. J. (2006). VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: Implications for vasculogenic mimicry. Cancer Biology & Therapy, 5(2), 228–233. https://doi.org/10.4161/cbt.5.2.2510
Paulis, Y. W., Soetekouw, P. M., Verheul, H. M., Tjan-Heijnen, V. C., & Griffioen, A. W. (2010). Signalling pathways in vasculogenic mimicry. Biochimica et Biophysica Acta, 1806(1), 18–28. https://doi.org/10.1016/j.bbcan.2010.01.001
Hendrix, M. J., Seftor, E. A., Meltzer, P. S., Gardner, L. M., Hess, A. R., Kirschmann, D. A., et al. (2001). Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proceedings of the National Academy of Sciences of the United States of America, 98(14), 8018–8023. https://doi.org/10.1073/pnas.131209798
Hess, A. R., Seftor, E. A., Gardner, L. M., Carles-Kinch, K., Schneider, G. B., Seftor, R. E., et al. (2001). Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: Role of epithelial cell kinase (Eck/EphA2). Cancer Research, 61(8), 3250–3255.
Margaryan, N. V., Strizzi, L., Abbott, D. E., Seftor, E. A., Rao, M. S., Hendrix, M. J., et al. (2009). EphA2 as a promoter of melanoma tumorigenicity. Cancer Biology & Therapy, 8(3), 279–288. https://doi.org/10.4161/cbt.8.3.7485
Sulzmaier, F. J., Jean, C., & Schlaepfer, D. D. (2014). FAK in cancer: Mechanistic findings and clinical applications. Nature Reviews Cancer, 14(9), 598–610. https://doi.org/10.1038/nrc3792
Lu, X. S., Sun, W., Ge, C. Y., Zhang, W. Z., & Fan, Y. Z. (2013). Contribution of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. International Journal of Oncology, 42(6), 2103–2115. https://doi.org/10.3892/ijo.2013.1897
Zhang, J. T., Sun, W., Zhang, W. Z., Ge, C. Y., Liu, Z. Y., Zhao, Z. M., et al. (2014). Norcantharidin inhibits tumor growth and vasculogenic mimicry of human gallbladder carcinomas by suppression of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer, 14, 193. https://doi.org/10.1186/1471-2407-14-193
Duxbury, M. S., Ito, H., Zinner, M. J., Ashley, S. W., & Whang, E. E. (2004). Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochemical and Biophysical Research Communications, 320(4), 1096–1102. https://doi.org/10.1016/j.bbrc.2004.06.054
Hess, A. R., & Hendrix, M. J. (2006). Focal adhesion kinase signaling and the aggressive melanoma phenotype. Cell Cycle, 5(5), 478–480. https://doi.org/10.4161/cc.5.5.2518
Fernandez-Cortes, M., Delgado-Bellido, D., & Oliver, F. J. (2019). Vasculogenic mimicry: Become an endothelial cell “but not so much.” Frontiers in Oncology, 9, 803. https://doi.org/10.3389/fonc.2019.00803
Delgado-Bellido, D., Fernández-Cortés, M., Rodríguez, M. I., Serrano-Sáenz, S., Carracedo, A., Garcia-Diaz, A., et al. (2019). VE-cadherin promotes vasculogenic mimicry by modulating kaiso-dependent gene expression. Cell Death and Differentiation, 26(2), 348–361. https://doi.org/10.1038/s41418-018-0125-4
Lissitzky, J. C., Parriaux, D., Ristorcelli, E., Vérine, A., Lombardo, D., & Verrando, P. (2009). Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Research, 69(3), 802–809. https://doi.org/10.1158/0008-5472.can-08-2391
Yousif, L. F., Di Russo, J., & Sorokin, L. (2013). Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhesion & Migration, 7(1), 101–110. https://doi.org/10.4161/cam.22680
Seftor, R. E., Seftor, E. A., Koshikawa, N., Meltzer, P. S., Gardner, L. M., Bilban, M., et al. (2001). Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Research, 61(17), 6322–6327.
Hess, A. R., Seftor, E. A., Seftor, R. E., & Hendrix, M. J. (2003). Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Research, 63(16), 4757–4762.
Sood, A. K., Fletcher, M. S., Coffin, J. E., Yang, M., Seftor, E. A., Gruman, L. M., et al. (2004). Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. American Journal of Obstetrics and Gynecology, 190(4), 899–909. https://doi.org/10.1016/j.ajog.2004.02.011
Winkler, F. (2017). Hostile takeover: How tumours hijack pre-existing vascular environments to thrive. The Journal of Pathology, 242(3), 267–272. https://doi.org/10.1002/path.4904
Hirschi, K. K., Rohovsky, S. A., & D’Amore, P. A. (1998). PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. Journal of Cell Biology, 141(3), 805–814. https://doi.org/10.1083/jcb.141.3.805
Yang, Z., Yao, H., Fei, F., Li, Y., Qu, J., Li, C., et al. (2018). Generation of erythroid cells from polyploid giant cancer cells: Re-thinking about tumor blood supply. Journal of Cancer Research and Clinical Oncology, 144(4), 617–627. https://doi.org/10.1007/s00432-018-2598-4
Liu, Q., Qiao, L., Liang, N., Xie, J., Zhang, J., Deng, G., et al. (2016). The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. Journal of Cellular and Molecular Medicine, 20(9), 1761–1769. https://doi.org/10.1111/jcmm.12851
Wagenblast, E., Soto, M., Gutiérrez-Ángel, S., Hartl, C. A., Gable, A. L., Maceli, A. R., et al. (2015). A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature, 520(7547), 358–362. https://doi.org/10.1038/nature14403
Lai, C. Y., Schwartz, B. E., & Hsu, M. Y. (2012). CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Research, 72(19), 5111–5118. https://doi.org/10.1158/0008-5472.can-12-0624
Zhao, H., Chen, S., & Fu, Q. (2020). Exosomes from CD133(+) cells carrying circ-ABCC1 mediate cell stemness and metastasis in colorectal cancer. Journal of Cellular Biochemistry, 121(5–6), 3286–3297. https://doi.org/10.1002/jcb.29600
Chen, L., Zhou, H., & Guan, Z. (2019). CircRNA_000543 knockdown sensitizes nasopharyngeal carcinoma to irradiation by targeting miR-9/platelet-derived growth factor receptor B axis. Biochemical and Biophysical Research Communications, 512(4), 786–792. https://doi.org/10.1016/j.bbrc.2019.03.126
Xiang, T., Lin, Y. X., Ma, W., Zhang, H. J., Chen, K. M., He, G. P., et al. (2018). Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nature Communications, 9(1), 5009. https://doi.org/10.1038/s41467-018-07308-5
Xu, S., Bai, J., Zhuan, Z., Li, B., Zhang, Z., Wu, X., et al. (2018). EBV-LMP1 is involved in vasculogenic mimicry formation via VEGFA/VEGFR1 signaling in nasopharyngeal carcinoma. Oncology Reports, 40(1), 377–384. https://doi.org/10.3892/or.2018.6414
David, J. M., Dominguez, C., Hamilton, D. H., & Palena, C. (2016). The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines (Basel), 4(3), https://doi.org/10.3390/vaccines4030022
Dwyer, J., Hebda, J. K., Le Guelte, A., Galan-Moya, E. M., Smith, S. S., Azzi, S., et al. (2012). Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS ONE, 7(9), e45562. https://doi.org/10.1371/journal.pone.0045562
Logue, S. E., McGrath, E. P., Cleary, P., Greene, S., Mnich, K., Almanza, A., et al. (2018). Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nature Communications, 9(1), 3267. https://doi.org/10.1038/s41467-018-05763-8
Mutalifu, N., Du, P., Zhang, J., Akbar, H., Yan, B., Alimu, S., et al. (2020). Circ_0000215 Increases the expression of CXCR2 and promoted the progression of glioma cells by sponging miR-495-3p. Technology in Cancer Research & Treatment, 19, 1533033820957026. https://doi.org/10.1177/1533033820957026
Seaman, S., Zhu, Z., Saha, S., Zhang, X. M., Yang, M. Y., Hilton, M. B., et al. (2017). Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell, 31(4), 501-515.e508. https://doi.org/10.1016/j.ccell.2017.03.005
Wang, L., Zhang, G. C., Kang, F. B., Zhang, L., & Zhang, Y. Z. (2019). hsa_circ0021347 as a potential target regulated by B7–H3 in modulating the malignant characteristics of osteosarcoma. BioMed Research International, 2019, 9301989. https://doi.org/10.1155/2019/9301989
Zeng, Y., Yao, X., Liu, X., He, X., Li, L., Liu, X., et al. (2019). Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. The Journal of Extracellular Vesicles, 8(1), 1629865. https://doi.org/10.1080/20013078.2019.1629865
Huang, X. Y., Huang, Z. L., Huang, J., Xu, B., Huang, X. Y., Xu, Y. H., et al. (2020). Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. Journal of Experimental & Clinical Cancer Research, 39(1), 20. https://doi.org/10.1186/s13046-020-1529-9
Rezaei, M., Martins Cavaco, A. C., Stehling, M., Nottebaum, A., Brockhaus, K., Caliandro, M. F., et al. (2020). Extracellular vesicle transfer from endothelial cells drives VE-cadherin expression in breast cancer cells, thereby causing heterotypic cell contacts. Cancers (Basel), 12(8), https://doi.org/10.3390/cancers12082138
Bergers, G., & Fendt, S. M. (2021). The metabolism of cancer cells during metastasis. Nature Reviews Cancer, 21(3), 162–180. https://doi.org/10.1038/s41568-020-00320-2
Zhang, H., & Lu, B. (2020). The roles of ceRNAs-mediated autophagy in cancer chemoresistance and metastasis. Cancers (Basel), 12(10), https://doi.org/10.3390/cancers12102926
Zang, J., Lu, D., & Xu, A. (2020). The interaction of circRNAs and RNA binding proteins: An important part of circRNA maintenance and function. Journal of Neuroscience Research, 98(1), 87–97. https://doi.org/10.1002/jnr.24356
Shang, Q., Yang, Z., Jia, R., & Ge, S. (2019). The novel roles of circRNAs in human cancer. Molecular Cancer, 18(1), 6. https://doi.org/10.1186/s12943-018-0934-6
Funding
This manuscript is supported by a grant from the National Natural Scientific Foundations of China (81872112).
Author information
Authors and Affiliations
Contributions
SY drafted the manuscript. LB conceived the idea, revised the manuscript, and approved the final submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shao, Y., Lu, B. The emerging roles of circular RNAs in vessel co-option and vasculogenic mimicry: clinical insights for anti-angiogenic therapy in cancers. Cancer Metastasis Rev 41, 173–191 (2022). https://doi.org/10.1007/s10555-021-10000-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-021-10000-8