Abstract
For more than 15 years, angiotropism in melanoma has been emphasized as a marker of extravascular migration of tumor cells along the abluminal vascular surface, unveiling an alternative mechanism of tumor spread distinct from intravascular dissemination. This mechanism has been termed extravascular migratory metastasis (EVMM). During EVMM, angiotropic tumor cells migrate in a ‘pericytic-like’ manner (pericytic mimicry) along the external surfaces of vascular channels, without intravasation. Through this pathway, melanoma cells may spread to nearby or more distant sites. Angiotropism is a prognostic factor predicting risk for metastasis in human melanoma, and a marker of EVMM in several experimental models. Importantly, analogies of EVMM and pericytic mimicry include neural crest cell migration, vasculogenesis and angiogenesis, and recent studies have suggested that the interaction between melanoma cells and the abluminal vascular surface induce differential expression of genes reminiscent of cancer migration and embryonic/stem cell state transitions. A recent work revealed that repetitive UV exposure of primary cutaneous melanomas in a genetically engineered mouse model promotes metastatic progression via angiotropism and migration along the abluminal vascular surface. Finally, recent data using imaging of melanoma cells in a murine model have shown the progression of tumor cells along the vascular surfaces. Taken together, these data provide support for the biological phenomenon of angiotropism and EVMM, which may open promising new strategies for reducing or preventing melanoma metastasis.





Similar content being viewed by others
References
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Orgaz JL, Sanz-Moreno V (2013) Emerging molecular targets in melanoma invasion and metastasis. Pigment Cell Melanoma Res 26:39–57
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65:9328–9337
Fukunaga-Kalabis M, Roesch A, Herlyn M (2011) From cancer stem cells to tumor maintenance in melanoma. J Invest Dermatol 131:1600–1604
Girouard SD, Murphy GF (2011) Melanoma stem cells: not rare, but well done. Lab Invest 91:647–664
Bailey CM, Morrison JA, Kulesa PM (2012) Melanoma revives an embryonic migration program to promote plasticity and invasion. Pigment Cell Melanoma Res 25:573–583
Wilder RJ (1956) The historical development of the concept of metastasis. J Mt Sinaï Hosp 23:728–734
Sleeman JP, Cady B, Pantel K (2012) The connectivity of lymphogenous and hematogenous tumor cell dissemination: biological insights and clinical implications. Clin Exp Metastasis 29:737–746
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70(14):5649–69, Jul 15
Paterlini-Bréchot P. (2011). Organ-specific markers in circulating tumor cell screening: an early indicator of metastasis-capable malignancy. Future Oncol. Jul;7 (7):849–71. doi: 10.2217/fon.11.32. Review. PubMed PMID: 21732757.
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Lugassy C, Eyden BP, Christensen L, Escande JP (1997) Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol 29(1):19–28
Lugassy C, Barnhill RL, Christensen L (2000) Melanoma and extravascular migratory metastasis. J Cutan Pathol 27(9):481
Lugassy C, Barnhill RL (2004) Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology 36:485–490
Lugassy C, Péault B, Wadehra M, Kleinman HK, Barnhill RL (2013) Could pericytic mimicry represent another type of melanoma cell plasticity with embryonic properties? PigmentCell Melanoma Res 26(5):746–54
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, Tüting T (2014) Ultraviolet radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507(7490):109–13
Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA (2008) Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol 32(9):1396–403
Levy MJ, Gleeson FC, Zhang L (2009) Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol 7(2):246
Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Slominski A (2008) Current concepts of metastasis in melanoma. Expert Rev Dermatol 3(5):569–585
Arias AM (2001) Epithelial mesenchymal interactions in cancer and development. Cell 105:425–431
Lugassy C, Barnhill RL. Angiotropic melanoma and extravascular migratory metastasis: a review. Adv Anat Pathol. May;14 (3):195–201 Review
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–90
Le Douarin NM, Creuzet S, Couly G, Dupin E (2004) Neural crest cell plasticity and its limits. Development 131:4637–4650
Perris R, Perissinotto D (2000) Role of the extracellular matrix during neural crest cell migration. Mech Dev 95:3–21
Schwarz Q, Maden CH, Vieira JM, Ruhrberg C (2009) Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. PNAS 106:6164–6169
Nagy N, Mwizerwa O, Yaniv K, Carmel L, Pieretti-Vanmarcke R, Weinstein BM, Goldstein AM (2009) Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev Biol 330:263–272
Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM (2007) Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7(4):246–55
Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ (2006) Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A 103:3752–3757
Weiss L (1986) Metastatic inefficiency: causes and consequences. Cancer Rev 3:1–24
Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283:139–146
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573
Fidler IJ (2002) The organ microenvironment and cancer metastasis. Differentiation 70:498–505
Drerup CM, Wiora HM, Topczewski J, Morris JA (2009) Disc1 regulates foxd3 and sox10 expression, affecting neural crest migration and differentiation. Development 136:2623–2632
Li A, Ma Y, Yu X et al (2011) Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod-driven motility and cell-cycle progression. Dev Cell 21:722–734
Hirschi KK, Rohovsky SA, D’Amore PA (1998) PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10 T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141:805–814
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Armulik A, Abramsson A, Betsholtz C (2005) Pericyte recruitment during angiogenesis. Circ Res 97:512–523
Crisan M, Yap S, Casteilla L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Yurchenco PD, Patton BL (2009) Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15:1277–1294
Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V (1997) Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277:225–228
Barnhill RL, Dy K, Lugassy C (2002) Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol 119:705–706
Wilmott J, Haydu L, Bagot M, Zhang Y, Jakrot V, McCarthy S, Lugassy C, Thompson J, Scolyer R, Barnhill R (2012) Angiotropism is an independent predictor of microscopic satellites in primary cutaneous melanoma. Histopathology 61:889–898
Lugassy C, Lazar V, Dessen P, van den Oord JJ, Winnepenninckx V, Spatz A, Bagot M, Bensussan A, Janin A, Eggermont AM, Barnhill RL (2011) Gene expression profiling of human angiotropic primary melanoma: selection of 15 differentially expressed genes potentially involved in extravascular migratory metastasis. Eur J Cancer 47:1267–1275
Lugassy C, Kleinman HK, Fernandez PM, Patierno SR, Webber MM, Ghanem G, Spatz A, Barnhill RL (2002) Human melanoma cell migration along capillary-like structures in vitro: a new dynamic model for studying extravascular migratory metastasis. J Invest Dermatol 119:703–704
Lugassy C, Kleinman HK, Engbring JA, Welch DR, Harms JF, Rufner R, Ghanem G, Patierno SR, Barnhill RL (2004) Angiotropism and pericyte-like location of GFP melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol 164:11911198
Zadran S, McMickle R, Shackelford D, Kleinman H, Barnhill R, Lugassy C (2013) Monitoring extra-vascular migratory metastasis (EVMM) of migrating cancer cells using an in vitro co-culture system. Protoc exch 22:2013
Lugassy C, Kleinman HK, Vernon SE, Welch DR, Barnhill RL (2007) C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br J Dermatol 157:780–782.6
Winnepenninckx V, Lazar V, Michiels S et al (2006) Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst 98(7):472–82
Dixon J, Jones NC, Sandell LL et al (2006) Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc Natl Acad Sci U S A 103(36):13403–8
Duband JL, Thiery JP (1987) Distribution of laminin and collagens during avian neural crest development. Development 101(3):461–78
Engbring JA, Kleinman HK (2003) Extracellular matrix and malignancy. J Pathol 200:465–470
Tzu J, Marinkovich MP (2008) Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol 40(2):199–214
Durbeej M (2010) Laminins. Cell Tissue Res 339(1):259–68
Lugassy C, Shahsafaei A, Bonitz P, Busam KJ, Barnhill RL (1999) Tumor microvessels in melanoma express the beta-2 chain of laminin Implications for melanoma metastasis. J Cutan Pathol 26:222–226
Lugassy C, Dickersin GR, Christensen L et al (1999) Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol 26:78–83
Nomizu M, Kuratomi Y, Song SY et al (1997) Identification of cell binding sequences in mouse laminin gamma1 chain by systematic peptide screening. J Biol Chem 272:32198–32205
Kuratomi Y, Nomizu M, Tanaka K et al (2002) Laminin gamma 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer 86:1169–1173
Lugassy C, Torres-Muñoz JE, Kleinman HK, Ghanem G, Vernon SE, Barnhill RL (2009) Over-expression of malignancy-associated laminins and laminin receptors by angiotropic human melanoma cells in a chick chorioallantoic membrane model. J Cutan Pathol 36(12):1237–43
Lugassy C, Wadehra M, Li X, Corselli M, Akhavan D, Binder SW, Péault B, Cochran AJ, Mischel PS, Kleinman HK, Barnhill RL (2013) Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron 6:19–29
Kubota Y, Kleinman HK, Martin GR, Lawley TJ (1988) Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 107:1589–1598
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155:739–752
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156(5):1002–16
Donnem T, Hu J, Ferguson M, Adighibe O, Snell C, Harris AL, Gatter KC, Pezzella F (2013) Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med 2(4):427–36
Shi H, Kong X, Ribas A, Lo RS (2011) Combinatorial treatments that overcome PDGFRb-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res 71:5067–5074
Lugassy C, Scolyer R, Long G, Menzies A, Mischel P, Barnhill RL (2014) PDGFBR expression in anti-BRAF resistant melanoma: are angiotropic melanoma cells a source of BRAF resistance and disease progression? J Cutan Pathol 41:159–160
Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ (2012) Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol 181:1115–1125
Barnhill RL, Busam KJ, From L, Bagot M, Lugassy C, Berwick M (2011) Inter-observer concordance for the recognition of angiotropism in human melanoma. Pigment Cell Melanoma Res 24(3):582–3
Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P (2006) Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53:799–808
Wesseling P, Ruiter DJ, Burger PC (1997) Angiogenesis in brain tumors: pathobiological and clinical aspects. J Neurooncol 32:253–265
Lugassy C, Haroun RI, Brem H, Tyler BM, Jones RV, Fernandez PM, Patierno SR, Kleinman HK, Barnhill RL (2002) Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol 24(6):473–8
Dirks PB (2001) Glioma migration: clues from the biology of neural progenitor cells and embryonic CNS cell migration. J Neurooncol 53:203–212
Suzuki SO, Kitai R, Llena J, Lee SC, Goldman JE, Shafit-Zagardo B (2002) MAP-2e, a novel MAP-2 isoform, is expressed in gliomas and delineates tumor architecture and patterns of infiltration. J Neuropathol Exp Neurol 61:403–412
Cheng L, Huang Z, Zhou W et al (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153:139–152
Lugassy C, Barnhill RL, Kleinman HK. (2013) Comment on line: Omission of references to prior work related to Cell publication: “Cheng et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth”. Cell. July http://www.cell.com/abstract/S0092-8674(13)00210-9#Comments.
Levy MJ, Gleeson FC, Zhang L (2009) Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol 7:246–248
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115(15):3379–91
Lugassy C, Vernon SE, Warner JW, Le CQ, Manyak M, Patierno SR, Barnhill RL (2005) Angiotropism of human prostate cancer cells: implications for extravascular migratory metastasis. BJU Int 95(7):1099–103
Dyke JM, Crook ML, Platten M, Stewart CJ (2014) Extravascular migratory metastasis in gynaecological carcinosarcoma. Histopathology 65(3):363–70
Mravic M, Asatrian G, Soo C, Lugassy C, Barnhill RL, Dry SM, Peault B, James AW (2014) From pericytes to perivascular tumors: correlates between pathology, stem cell biology, and tissue engineering. Int Orthop 38(9):1819–24
Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F (2008) PEComas: the past, the present and the future. Virchows Arch 452(2):119–32
Hirayama R, Sato K, Hirokawa K, Chang MP, Mishima Y, Makinodan T (1984) Different metastatic modes of malignant melanoma implanted in the ear of young and old mice. Cancer Immunol Immunother 18(3):209–14
Acknowledgments
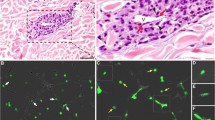
In vivo confocal imaging shown in Fig. 5 was performed at the California NanoSystems Institute (CNSI) Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA with support from a NIH/National Center for Advancing Translational Science UCLA CTSI Grant (UL1TR000124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lugassy, C., Zadran, S., Bentolila, L.A. et al. Angiotropism, Pericytic Mimicry and Extravascular Migratory Metastasis in Melanoma: An Alternative to Intravascular Cancer Dissemination. Cancer Microenvironment 7, 139–152 (2014). https://doi.org/10.1007/s12307-014-0156-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-014-0156-4