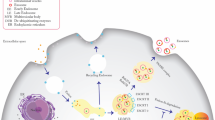
Abstract
Cancer-initiating cells (CIC) are the driving force in tumor progression. There is strong evidence that CIC fulfill this task via exosomes (TEX), which modulate and reprogram stroma, nontransformed cells, and non-CIC. Characterization of CIC, besides others, builds on expression of CIC markers, many of which are known as metastasis-associated molecules. We here discuss that the linkage between CIC/CIC-TEX and metastasis-associated molecules is not fortuitously, but relies on the contribution of these markers to TEX biogenesis including loading and TEX target interactions. In addition, CIC markers contribute to TEX binding- and uptake-promoted activation of signaling cascades, transcription initiation, and translational control. Our point of view will be outlined for pancreas and colon CIC highly expressing CD44v6, Tspan8, EPCAM, claudin7, and LGR5, which distinctly but coordinately contribute to tumor progression. Despite overwhelming progress in unraveling the metastatic cascade and the multiple tasks taken over by CIC-TEX, there remains a considerable gap in linking CIC biomarkers, TEX, and TEX-initiated target modulation with metastasis. We will try to outline possible bridges, which could allow depicting pathways for new and expectedly powerful therapeutic interference with tumor progression.







Similar content being viewed by others
Notes
Full names of proteins and genes are listed in Table S1.
References
Steck, P. A., North, S. M., & Nicolson, G. L. (1987). Purification and partial characterization of a tumour-metastasis-associated high-Mr glycoprotein from rat 13762NF mammary adenocarcinoma cells. The Biochemical Journal, 242(3), 779–787.
Raz, A., Pazerini, G., & Carmi, P. (1989). Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Research, 49(13), 3489–3493.
Rao, C. N., Castronovo, V., Schmitt, M. C., Wewer, U. M., Claysmith, A. P., Liotta, L. A., et al. (1989). Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry, 28(18), 7476–7486.
Stewart, R. L., & O'Connor, K. L. (2015). Clinical significance of the integrin α6β4 in human malignancies. Laboratory Investigation, 95(9), 976–986. https://doi.org/10.1038/labinvest.2015.82.
Günthert, U., Hofmann, M., Rudy, W., Reber, S., Zöller, M., Haussmann, I., et al. (1991). A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell, 65(1), 13–24.
Toh, Y., Pencil, S. D., & Nicolson, G. L. (1994). A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. Journal of Biological Chemistry, 269(37), 22958–22963.
Kaur, E., Gupta, S., & Dutt, S. (2014). Clinical implications of MTA proteins in human cancer. Cancer Metastasis Reviews, 33(4), 1017–1024. https://doi.org/10.1007/s10555-014-9527-z.
Malisetty, V. L., Penugurti, V., Panta, P., Chitta, S. K., & Manavathi, B. (2017). MTA1 expression in human cancers—clinical and pharmacological significance. Biomedicine & Pharmacotherapy, 95, 956–964. https://doi.org/10.1016/j.biopha.2017.09.025.
Karhemo, P. R., Hyvönen, M., & Laakkonen, P. (2012). Metastasis-associated cell surface oncoproteomics. Frontiers in Pharmacology, 3, 192. https://doi.org/10.3389/fphar.2012.00192.
Zhang, Y. Y., Chen, B., & Ding, Y. Q. (2012). Metastasis-associated factors facilitating the progression of colorectal cancer. Asian Pacific Journal of Cancer Prevention, 13(6), 2437–2244.
Gupta, P. B., Mani, S., Yang, J., Hartwell, K., & Weinberg, R. A. (2005). The evolving portrait of cancer metastasis. Cold Spring Harbor Symposia on Quantitative Biology, 70, 291–297.
Dexter, T. M. (1979). Haemopoiesis in long-term bone marrow cultures. A review. Acta Haematologica, 62(5–6), 299–305.
Leventhal, B. G., & Konior, G. S. (1976). Leukemia: a critical review. Seminars in Oncology, 3(3), 319–325.
Ailles, L. E., & Weissman, I. L. (2007). Cancer stem cells in solid tumors. Current Opinion in Biotechnology, 18(5), 460–466.
Tirino, V., Desiderio, V., Paino, F., De Rosa, A., Papaccio, F., La Noce, M., et al. (2013). Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. The FASEB Journal, 27(1), 13–24. https://doi.org/10.1096/fj.12-218222.
Brabletz, T., Kalluri, R., Nieto, M. A., & Weinberg, R. A. (2018). EMT in cancer. Nature Reviews. Cancer, 18(2), 128–134. https://doi.org/10.1038/nrc.2017.118.
Woodward, W. A., & Sulman, E. P. (2008). Cancer stem cells: markers or biomarkers? Cancer Metastasis Reviews, 27(3), 459–470. https://doi.org/10.1007/s10555-008-9130-2.
Keysar, S. B., & Jimeno, A. (2010). More than markers: biological significance of cancer stem cell-defining molecules. Molecular Cancer Therapeutics, 9(9), 2450–2457. https://doi.org/10.1158/1535-7163.MCT-10-0530.
Murar, M., & Vaidya, A. (2015). Cancer stem cell markers: premises and prospects. Biomarkers in Medicine, 9(12), 1331–1342. https://doi.org/10.2217/bmm.15.85.
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L., & Turbide, C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of Biological Chemistry, 262(19), 9412–9420.
Rashed, M. H., Bayraktar, E., Helal, G. K., Abd-Ellah, M. F., Amero, P., Chavez-Reyes, A., et al. (2017). Exosomes: from garbage bins to promising therapeutic targets. International Journal of Molecular Sciences, 18(3), E538. https://doi.org/10.3390/ijms18030538.
Lobb, R. J., Lima, L. G., & Möller, A. (2017). Exosomes: key mediators of metastasis and pre-metastatic niche formation. Seminars in Cell & Developmental Biology, 67, 3–10. https://doi.org/10.1016/j.semcdb.2017.01.004.
Steinbichler, T. B., Dudás, J., Riechelmann, H., & Skvortsova, I. I. (2017). The role of exosomes in cancer metastasis. Seminars in Cancer Biology, 44, 170–181. https://doi.org/10.1016/j.semcancer.2017.02.006.
Wu, J., Qu, Z., Fei, Z. W., Wu, J. H., & Jiang, C. P. (2017). Role of stem cell-derived exosomes in cancer. Oncology Letters, 13(5), 2855–2866. https://doi.org/10.3892/ol.2017.5824.
Sharma, A. (2018). Role of stem cell derived exosomes in tumor biology. International Journal of Cancer, 142(6), 1086–1092. https://doi.org/10.1002/ijc.31089.
Sato, S., & Weaver, A. M. (2018). Extracellular vesicles: important collaborators in cancer progression. Essays in Biochemistry, 62(2), 149–163. https://doi.org/10.1042/EBC20170080.
Abak, A., Abhari, A., & Rahimzadeh, S. (2018). Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ, 6, e4763. https://doi.org/10.7717/peerj.4763.
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: a Cancer Journal for Clinicians, 66(1), 7–30. https://doi.org/10.3322/caac.21332.
Engelhardt, E. G., Révész, D., Tamminga, H. J., Punt, C. J. A., Koopman, M., Onwuteaka-Philipsen, B. D., et al. (2018). Clinical usefulness of tools to support decision-making for palliative treatment of metastatic colorectal cancer: a systematic review. Clinical Colorectal Cancer, 17(1), e1–e12. https://doi.org/10.1016/j.clcc.2017.06.007.
Brenner, H., Kloor, M., & Pox, C. P. (2014). Colorectal cancer. Lancet, 383, 1490–1502.
Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., Murray, T., et al. (2008). Cancer statistics. CA: a Cancer Journal for Clinicians, 58(2), 71–96.
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J. W., Comber, H., et al. (2013). Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European Journal of Cancer, 49(6), 1374–1403. https://doi.org/10.1016/j.ejca.2012.12.027.
Ahrendt, S. A., & Pitt, H. A. (2002). Surgical management of pancreatic cancer. Oncology (Williston Park), 16(6), 725–734 discussion 734, 736–728, 740, 743.
Rahib, L., Smith, B. D., Aizenberg, R., Rosenzweig, A. B., Fleshman, J. M., & Matrisian, L. M. (2014). Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research, 74(11), 2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155.
Del Chiaro, M., Segersvärd, R., Lohr, M., & Verbeke, C. (2014). Early detection and prevention of pancreatic cancer: is it really possible today? World Journal of Gastroenterology, 20, 12118–12131.
Ajani, J. A., Song, S., Hochster, H. S., & Steinberg, I. B. (2015). Cancer stem cells: the promise and the potential. Seminars in Oncology, 42(Suppl 1), S3–S17.
Weinstein, I. B. (1987). Growth factors, oncogenes, and multistage carcinogenesis. Journal of Cellular Biochemistry, 33(3), 213–224.
Hong, S. N. (2018). Genetic and epigenetic alterations of colorectal cancer. Intest Res, 16(3), 327–337. https://doi.org/10.5217/ir.2018.16.3.327.
Aguirre, A. J., & Collisson, E. A. (2017). Advances in the genetics and biology of pancreatic cancer. Cancer Journal, 23(6), 315–320. https://doi.org/10.1097/PPO.0000000000000286.
Shiozawa, Y., Nie, B., Pienta, K. J., Morgan, T. M., & Taichman, R. S. (2013). Cancer stem cells and their role in metastasis. Pharmacology & Therapeutics, 138(2), 285–293. https://doi.org/10.1016/j.pharmthera.2013.01.014.
Li, S., & Li, Q. (2014). Cancer stem cells and tumor metastasis (review). International Journal of Oncology, 44(6), 1806–1812. https://doi.org/10.3892/ijo.2014.2362.
Daley, G. Q. (2015). Stem cells and the evolving notion of cellular identity. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 370(1680), 20140376. https://doi.org/10.1098/rstb.2014.0376.
Forsberg, E. C., Bhattacharya, D., & Weissman, I. L. (2006). Hematopoietic stem cells: expression profiling and beyond. Stem Cell Reviews, 2(1), 23–30.
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J., & Campbell, K. H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385(6619), 810–813.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Park, I. H., Zhao, R., West, J. A., Yabuuchi, A., Huo, H., Ince, T. A., et al. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451(7175), 141–146.
Jonsson, J., Carlsson, L., Edlund, T., & Edlund, H. (1994). Insulin-promoter-factor 1 is required for pancreas development in mice. Nature, 371(6498), 606–609.
Gu, G., Dubauskaite, J., & Melton, D. A. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development, 129(10), 2447–2457.
Rostovskaya, M., Bredenkamp, N., & Smith, A. (2015). Towards consistent generation of pancreatic lineage progenitors from human pluripotent stem cells. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 370(1680), 20140365. https://doi.org/10.1098/rstb.2014.0365.
Jiang, F. X., & Morahan, G. (2014). Pancreatic stem cells remain unresolved. Stem Cells and Development, 23(23), 2803–2812. https://doi.org/10.1089/scd.2014.0214.
Larsen, H. L., & Grapin-Botton, A. (2017). The molecular and morphogenetic basis of pancreas organogenesis. Seminars in Cell & Developmental Biology, 66, 51–68. https://doi.org/10.1016/j.semcdb.2017.01.005.
Sznurkowska, M. K., Hannezo, E., Azzarelli, R., Rulands, S., Nestorowa, S., Hindley, C. J., et al. (2018). Defining lineage potential and fate behavior of precursors during pancreas development. Developmental Cell, 46(3), 360–375.e5. https://doi.org/10.1016/j.devcel.2018.06.028.
Buczacki, S. J., Zecchini, H. I., Nicholson, A. M., Russell, R., Vermeulen, L., Kemp, R., et al. (2013). Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature, 495(7439), 65–69. https://doi.org/10.1038/nature11965.
Zhang, Z., & Huang, J. (2013). Intestinal stem cells—types and markers. Cell Biology International, 37(5), 406–414. https://doi.org/10.1002/cbin.10049.
Clevers, H. C., & Bevins, C. L. (2013). Paneth cells: maestros of the small intestinal crypts. Annual Review of Physiology, 75, 289–311. https://doi.org/10.1146/annurev-physiol-030212-183744.
Kriz, V., & Korinek, V. (2018). Wnt, RSPO and Hippo signalling in the intestine and intestinal stem cells. Genes (Basel), 9(1), E20. https://doi.org/10.3390/genes9010020.
Krausova, M., & Korinek, V. (2014). Wnt signaling in adult intestinal stem cells and cancer. Cellular Signalling, 26(3), 570–579. https://doi.org/10.1016/j.cellsig.2013.11.032.
Clevers, H., Loh, K. M., & Nusse, R. (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 346(6205), 1248012. https://doi.org/10.1126/science.1248012.
Park, S., Cui, J., Yu, W., Wu, L., Carmon, K. S., & Liu, Q. J. (2018). Differential activities and mechanisms of the four R-spondins in potentiating Wnt/β-catenin signaling. The Journal of Biological Chemistry, 293(25), 9759–9769. https://doi.org/10.1074/jbc.RA118.002743.
Yan, K. S., Janda, C. Y., Chang, J., Zheng, G. X. Y., Larkin, K. A., Luca, V. C., et al. (2017). Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature, 545(7653), 238–242. https://doi.org/10.1038/nature22313.
Passegué, E., & Weisman, I. L. (2005). Leukemic stem cells: where do they come from? Stem Cell Reviews, 1(3), 181–188.
Johnsen, H. E., Kjeldsen, M. K., Urup, T., Fogd, K., Pilgaard, L., Boegsted, M., et al. (2009). Cancer stem cells and the cellular hierarchy in haematological malignancies. European Journal of Cancer, 45(Suppl 1), 194–201. https://doi.org/10.1016/S0959-8049(09)70033-4.
Shah, M., & Allegrucci, C. (2013). Stem cell plasticity in development and cancer: epigenetic origin of cancer stem cells. Sub-Cellular Biochemistry, 61, 545–565. https://doi.org/10.1007/978-94-007-4525-4_24.
Verga Falzacappa, M. V., Ronchini, C., Reavie, L. B., & Pelicci, P. G. (2012). Regulation of self-renewal in normal and cancer stem cells. The FEBS Journal, 279(19), 3559–3572. https://doi.org/10.1111/j.1742-4658.2012.08727.x.
Liu, J. (2018). The dualistic origin of human tumors. Seminars in Cancer Biology, 2018. https://doi.org/10.1016/j.semcancer.2018.07.004.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–111.
Mantamadiotis, T., & Taraviras, S. (2011). Self-renewal mechanisms in neural cancer stem cells. Front Biosci (Landmark Ed), 16, 598–607.
Hinge, A., & Filippi, M. D. (2016). Deconstructing the complexity of TGFβ signaling in hematopoietic stem cells: quiescence and beyond. Curr Stem Cell Rep, 2(4), 388–397. https://doi.org/10.1007/s40778-016-0069-x.
Soteriou, D., & Fuchs, Y. (2018). A matter of life and death: stem cell survival in tissue regeneration and tumour formation. Nature Reviews. Cancer, 18(3), 187–201. https://doi.org/10.1038/nrc.2017.122.
Alison, M. R., Guppy, N. J., Lim, S. M., & Nicholson, L. J. (2010). Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? The Journal of Pathology, 222(4), 335–344. https://doi.org/10.1002/path.2772.
Easwaran, H., Tsai, H. C., & Baylin, S. B. (2014). Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Molecular Cell, 54(5), 716–727. https://doi.org/10.1016/j.molcel.2014.05.015.
Colak, S., & Medema, J. P. (2014). Cancer stem cells—important players in tumor therapy resistance. The FEBS Journal, 281(21), 4779–4791. https://doi.org/10.1111/febs.13023.
Skvortsova, I., Debbage, P., Kumar, V., & Skvortsov, S. (2015). Radiation resistance: cancer stem cells (CSCs) and their enigmatic pro-survival signaling. Seminars in Cancer Biology, 35, 39–44. https://doi.org/10.1016/j.semcancer.2015.09.009.
Lipinska, N., Romaniuk, A., Paszel-Jaworska, A., Toton, E., Kopczynski, P., & Rubis, B. (2017). Telomerase and drug resistance in cancer. Cellular and Molecular Life Sciences, 74(22), 4121–4132. https://doi.org/10.1007/s00018-017-2573-2.
Yan, Y., Zuo, X., & Wie, D. (2015). Concise review: Emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Translational Medicine, 4(9), 1033–1043. https://doi.org/10.5966/sctm.2015-0048.
de Lucas, B., Pérez, L. M., & Gálvez, B. G. (2018). Importance and regulation of adult stem cell migration. Journal of Cellular and Molecular Medicine, 22(2), 746–754. https://doi.org/10.1111/jcmm.13422.
Hamidi, H., & Ivaska, J. (2018). Every step of the way: integrins in cancer progression and metastasis. Nature Reviews. Cancer. https://doi.org/10.1038/s41568-018-0038-z.
Smith, G. H., & Boulanger, C. A. (2003). Mammary epithelial stem cells: transplantation and self-renewal analysis. Cell Proliferation, 36(Suppl 1), 3–15.
Whittle, J. R., Lewis, M. T., Lindeman, G. J., & Visvader, J. E. (2015). Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Research, 17, 17. https://doi.org/10.1186/s13058-015-0523-1.
Lapidot, T., Sirard, C., Vormoor, J., Murdoch, B., Hoang, T., Caceres-Cortes, J., et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature, 367(6464), 645–648.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100(7), 3983–3988.
Moghbeli, M., Moghbeli, F., Forghanifard, M. M., & Abbaszadegan, M. R. (2014). Cancer stem cell detection and isolation. Medical Oncology, 31(9), 69. https://doi.org/10.1007/s12032-014-0069-6.
Telford, W. G. (2013). Stem cell identification by DyeCycle Violet side population analysis. Methods in Molecular Biology, 946, 163–179. https://doi.org/10.1007/978-1-62703-128-8_11.
Ishiguro, T., Ohata, H., Sato, A., Yamawaki, K., Enomoto, T., & Okamoto, K. (2017). Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Science, 108(3), 283–289. https://doi.org/10.1111/cas.13155.
Ma, I., & Allan, A. L. (2011). The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Reviews, 7(2), 292–306. https://doi.org/10.1007/s12015-010-9208-4.
Duan, J. J., Cai, J., Guo, Y. F., Bian, X. W., & Yu, S. C. (2016). ALDH1A3, a metabolic target for cancer diagnosis and therapy. International Journal of Cancer, 139(5), 965–975. https://doi.org/10.1002/ijc.30091.
Mele, L., Liccardo, D., & Tirino, V. (2018). Evaluation and isolation of cancer stem cells using ALDH activity assay. Methods in Molecular Biology, 1692, 43–48. https://doi.org/10.1007/978-1-4939-7401-6_4.
Gopalan, V., Islam, F., & Lam, A. K. (2018). Surface markers for the identification of cancer stem cells. Methods in Molecular Biology, 1692, 17–29. https://doi.org/10.1007/978-1-4939-7401-6_2.
Pelosi, E., Castelli, G., & Testa, U. (2015). Targeting LSCs through membrane antigens selectively or preferentially expressed on these cells. Blood Cells, Molecules & Diseases, 55(4), 336–346. https://doi.org/10.1016/j.bcmd.2015.07.015.
Bao, B., Ahmad, A., Azmi, A.S., Ali, S., & Sarkar, F.H. (2013). Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol, Chapter 14:Unit 14.25. doi: 10.1002/0471141755.ph1425s61.
Guzman, M. L., & Allan, J. N. (2014). Concise review: Leukemia stem cells in personalized medicine. Stem Cells, 32(4), 844–851. https://doi.org/10.1002/stem.1597.
Munz, M., Baeuerle, P. A., & Gires, O. (2009). The emerging role of EpCAM in cancer and stem cell signaling. Cancer Research, 69(14), 5627–5629. https://doi.org/10.1158/0008-5472.CAN-09-0654.
Todaro, M., Gaggianesi, M., Catalano, V., Benfante, A., Iovino, F., Biffoni, M., et al. (2014). CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell, 14(3), 342–356. https://doi.org/10.1016/j.stem.2014.01.009.
Smith, N. R., Davies, P. S., Levin, T. G., Gallagher, A. C., Keene, D. R., Sengupta, S. K., et al. (2017). Cell adhesion molecule CD166/ALCAM functions within the crypt to orchestrate murine intestinal stem cell homeostasis. Cellular and Molecular Gastroenterology and Hepatology, 3(3), 389–409. https://doi.org/10.1016/j.jcmgh.2016.12.010.
Jung, P., Sato, T., Merlos-Suárez, A., Barriga, F. M., Iglesias, M., Rossell, D., et al. (2011). Isolation and in vitro expansion of human colonic stem cells. Nature Medicine, 17(10), 1225–1227. https://doi.org/10.1038/nm.2470.
Li, C., Wu, J. J., Hynes, M., Dosch, J., Sarkar, B., Welling, T. H., et al. (2011). c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology, 141(6), 2218–2227.e5. https://doi.org/10.1053/j.gastro.2011.08.009.
Hermann, P. C., Huber, S. L., Herrler, T., Aicher, A., Ellwart, J. W., Guba, M., et al. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1(3), 313–323. https://doi.org/10.1016/j.stem.2007.06.002.
Dalerba, P., Dylla, S. J., Park, I. K., Liu, R., Wang, X., Cho, R. W., et al. (2007). Phenotypic characterization of human colorectal cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104(24), 10158–10163.
Ricci-Vitiani, L., Lombardi, D. G., Pilozzi, E., Biffoni, M., Todaro, M., Peschle, C., et al. (2007). Identification and expansion of human colon-cancer-initiating cells. Nature, 445(7123), 111–115.
Ren, F., Sheng, W. Q., & Du, X. (2013). CD133: a cancer stem cells marker, is used in colorectal cancers. World Journal of Gastroenterology, 19(17), 2603–2611. https://doi.org/10.3748/wjg.v19.i17.2603.
Mak, A. B., Nixon, A. M., Kittanakom, S., Stewart, J. M., Chen, G. I., Curak, J., et al. (2012). Regulation of CD133 by HDAC6 promotes β-catenin signaling to suppress cancer cell differentiation. Cell Reports, 2(4), 951–963. https://doi.org/10.1016/j.celrep.2012.09.016.
Shimozato, O., Waraya, M., Nakashima, K., Souda, H., Takiguchi, N., Yamamoto, H., et al. (2015). Receptor-type protein tyrosine phosphatase κ directly dephosphorylates CD133 and regulates downstream AKT activation. Oncogene, 34(15), 1949–1960. https://doi.org/10.1038/onc.2014.141.
Röper, K., Corbeil, D., & Huttner, W. B. (2000). Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nature Cell Biology, 2(9), 582–592.
Giebel, B., Corbeil, D., Beckmann, J., Höhn, J., Freund, D., Giesen, K., et al. (2004). Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood, 104(8), 2332–2338.
Simons, K., & Toomre, D. (2000). Lipid rafts and signal transduction. Nature Reviews. Molecular Cell Biology, 1(1), 31–39.
Fonseca, A. V., Bauer, N., & Corbeil, D. (2008). The stem cell marker CD133 meets the endosomal compartment—new insights into the cell division of hematopoietic stem cells. Blood Cells, Molecules & Diseases, 41(2), 194–195. https://doi.org/10.1016/j.bcmd.2008.04.004.
Kemper, K., Prasetyanti, P. R., De Lau, W., Rodermond, H., Clevers, H., & Medema, J. P. (2012). Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells, 30(11), 2378–2386. https://doi.org/10.1002/stem.1233.
de Lau, W., Barker, N., Low, T. Y., Koo, B. K., Li, V. S., Teunissen, H., et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature, 476(7360), 293–297. https://doi.org/10.1038/nature10337.
Koo, B. K., & Clevers, H. (2014). Stem cells marked by the R-spondin receptor LGR5. Gastroenterology, 147(2), 289–302. https://doi.org/10.1053/j.gastro.2014.05.007.
de Sousa e Melo, F., Kurtova, A. V., Harnoss, J. M., Kljavin, N., Hoeck, J. D., Hung, J., et al. (2017). A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature, 543(7647), 676–680. https://doi.org/10.1038/nature21713.
Leung, C., Tan, S. H., & Barker, N. (2018). Recent advances in Lgr5+ stem cell research. Trends in Cell Biology, 28(5), 380–391. https://doi.org/10.1016/j.tcb.2018.01.010.
Idzerda, R. L., Carter, W. G., Nottenburg, C., Wayner, E. A., Gallatin, W. M., & John, T. (1989). Isolation and DNA sequence of a cDNA clone encoding a lymphocyte adhesion receptor for high endothelium. Proceedings of the National Academy of Sciences of the United States of America, 86, 4659–4663.
Goldstein, L. A., & Butcher, E. C. (1990). Identification of mRNA that encodes an alternative form of H-CAM (CD44) in lymphoid and nonlymphoid tissues. Immunogenetics, 32, 389–397.
Screaton, G. R., Bell, M. V., Jackson, D. G., Cornelis, F. B., Gerth, U., & Bell, J. I. (1992). Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proceedings of the National Academy of Sciences of the United States of America, 89, 12160–12164.
Ishii, S., Ford, R., Thomas, P., Nachman, A., Steele, G., Jr., & Jessup, J. M. (1993). CD44 participates in the adhesion of human colorectal carcinoma cells to laminin and type IV collagen. Surgical Oncology, 2, 255–264.
Bennett, K. L., Jackson, D. G., Simon, J. C., Tanczos, E., Peach, R., Modrell, B., et al. (1995). CD44 isoforms containing exon v3 are responsible for the presentation of heparin-binding growth factor. The Journal of Cell Biology, 128, 687–698.
Neame, S. J., & Isacke, C. M. (1993). The cytoplasmic tail of CD44 is required for basolateral localization in ephitelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. The Journal of Cell Biology, 121, 1299–1310.
Liu, D., & Sy, M. S. (1997). Phorbol myristate acetate stimulates the dimerization of CD44 involving a cysteine in the transmembrane domain. Journal of Immunology, 159, 2702–2711.
Föger, N., Marhaba, R., & Zöller, M. (1999). Raft associated interaction of CD44 with the cytoskeleton. Journal of Cell Science, 114, 1169–1178.
Oliferenko, S., Paiha, K., Harder, T., Gerke, V., Schwärzler, C., Schwarz, H., et al. (1999). Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. The Journal of Cell Biology, 146, 843–854.
Lokeshwar, V. B., Fregien, N., & Bourguignon, L. Y. (1994). Ankyrin-binding domain of CD44(Gp85) is required for the expression of hyaluronic acid-mediated adhesion function. The Journal of Cell Biology, 126, 1099–1109.
Ruiz, P., Schwärzler, C., & Günthert, U. (1995). CD44 isoforms during differentiation and development. Bioessays, 17, 17–24.
Jalkanen, S., & Jalkanen, M. (1992). Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. The Journal of Cell Biology, 116, 817–825.
Toyama-Sorimachi, N., & Miyasaka, M. (1994). A novel ligand for CD44 is sulfated proteoglycan. International Immunology, 6, 655–660.
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B., & Seed, B. (1990). CD44 is the principal cell surface receptor for hyaluronate. Cell, 61, 1303–1313.
Greenfield, B., Wang, W. C., Marquardt, H., Piepkorn, M., Wolff, E. A., Aruffo, A., et al. (1999). Characterization of the heparan sulfate and chondroitin sulfate assembly sites in CD44. The Journal of Biological Chemistry, 274, 2511–2517.
Higman, V. A., Briggs, D. C., Mahoney, D. J., Blundell, C. D., Sattelle, B. M., Dyer, D. P., et al. (2014). A refined model for the TSG-6 link module in complex with hyaluronan: use of defined oligosaccharides to probe structure and function. The Journal of Biological Chemistry, 289, 5619–5634. https://doi.org/10.1074/jbc.M113.542357.
Orian-Rousseau, V., & Ponta, H. (2008). Adhesion proteins meet receptors: a common theme? Advances in Cancer Research, 101, 63–92.
Tremmel, M., Matzke, A., Albrecht, I., Laib, A. M., Olaku, V., Ballmer-Hofer, K., et al. (2009). A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood, 114, 5236–5244. https://doi.org/10.1182/blood-2009-04-219204.
Kim, M. S., Park, M. J., Moon, E. J., Kim, S. J., Lee, C. H., Yoo, H., et al. (2005). Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to enhance the motility of human glioma cells. Cancer Research, 65, 686–691.
Orian-Rousseau, V. (2015). CD44 acts as a signaling platform controlling tumor progression and metastasis. Frontiers in Immunology, 6, 154. https://doi.org/10.3389/fimmu.2015.00154.
Mori, T., Kitano, K., Terawaki, S., Maesaki, R., Fukami, Y., & Hakoshima, T. (2008). Structural basis for CD44 recognition by ERM proteins. The Journal of Biological Chemistry, 283, 29602–29612.
Fehon, R. G., McClatchey, A. I., & Bretscher, A. (2010). Organizing the cell cortex: the role of ERM proteins. Nature Reviews. Molecular Cell Biology, 11, 276–287.
Stamenkovic, I., & Yu, Q. (2010). Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell. Motility, proliferation, and survival. Current Protein & Peptide Science, 11, 471–484.
Orian-Rousseau, V., Morrison, H., Matzke, A., Kastilan, T., Pace, G., Herrlich, P., et al. (2007). Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Molecular Biology of the Cell, 18, 76–83. https://doi.org/10.1091/mbc.E06-08-0674.
Adamia, S., Maxwell, C. A., & Pilarski, L. M. (2005). Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Current Drug Targets. Cardiovascular & Haematological Disorders, 5, 3–14.
Misra, S., Toole, B. P., & Ghatak, S. (2006). Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. The Journal of Biological Chemistry, 281, 34936–34941.
Kozovska, Z., Gabrisova, V., & Kucerova, L. (2014). Colon cancer: cancer stem cells markers, drug resistance and treatment. Biomedicine & Pharmacotherapy, 68, 911–916. https://doi.org/10.1016/j.biopha.2014.10.019.
Grass, G. D., Dai, L., Qin, Z., Parsons, C., & Toole, B. P. (2014). CD147: regulator of hyaluronan signaling in invasiveness and chemoresistance. Advances in Cancer Research, 123, 351–373. https://doi.org/10.1016/B978-0-12-800092-2.00013-7.
Bourguignon, L. Y. (2008). Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Seminars in Cancer Biology, 18, 251–259.
Ghatak, S., Misra, S., & Toole, B. P. (2005). Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. The Journal of Biological Chemistry, 280, 8875–8883. https://doi.org/10.1074/jbc.M410882200.
Heldin, P., Basu, K., Kozlova, I., & Porsch, H. (2014). HAS2 and CD44 in breast tumorigenesis. Advances in Cancer Research, 123, 211–229. https://doi.org/10.1016/B978-0-12-800092-2.00008-3.
Xu, H., Tian, Y., Yuan, X., Wu, H., Liu, Q., Pestell, R. G., et al. (2015). The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther, 8, 3783–3792. https://doi.org/10.2147/OTT.S95470.
Nastase, M. V., Janicova, A., Wygrecka, M., & Schaefer, L. (2017). Signaling at the crossroads: matrix-derived proteoglycan and reactive oxygen species signaling. Antioxidants & Redox Signaling, 27(12), 855–873. https://doi.org/10.1089/ars.2017.7165.
Ekyalongo, R. C., Nakayama, H., Kina, K., Kaga, N., & Iwabuchi, K. (2015). Organization and functions of glycolipid-enriched microdomains in phagocytes. Biochimica et Biophysica Acta, 1851, 90–97. https://doi.org/10.1016/j.bbalip.2014.06.009.
Korcsmaros, T., & Schneider, M. V. (2017). Superti-Furga G. Next generation of network medicine: interdisciplinary signaling approaches. Integr Biol (Camb), 9, 97–108. https://doi.org/10.1039/c6ib00215c.
Stipp, C. S., Kolesnikova, T. V., & Hemler, M. E. (2003). Functional domains in tetraspanin proteins. Trends in Biochemical Sciences, 28, 106–112.
Hemler, M. E. (2005). Tetraspanin functions and associated microdomains. Nature Reviews. Molecular Cell Biology, 6, 801–811.
Levy, S., & Shoham, T. (2005). Protein-protein interactions in the tetraspanin web. Physiology (Bethesda), 20, 218–224.
Halova, I., & Draber, P. (2016). Tetraspanins and transmembrane adaptor proteins as plasma membrane organizers—mast cell case. Frontiers in Cell and Development Biology, 4, 43. https://doi.org/10.3389/fcell.2016.00043.
Berditchevski, F., & Odintsova, E. (2007). Tetraspanins as regulators of protein trafficking. Traffic, 8, 89–96.
Yáñez-Mó, M., Gutiérrez-López, M. D., & Cabañas, C. (2011). Functional interplay between tetraspanins and proteases. Cellular and Molecular Life Sciences, 68, 3323–3335. https://doi.org/10.1007/s00018-011-0746-y.
Stepanek, O., Draber, P., & Horejsi, V. (2014). Palmitoylated transmembrane adaptor proteins in leukocyte signaling. Cellular Signalling, 26, 895–902. https://doi.org/10.1016/j.cellsig.2014.01.007.
Termini, C. M., & Gillette, J. M. (2017). Tetraspanins function as regulators of cellular signaling. Frontiers in Cell and Development Biology, 5, 34. https://doi.org/10.3389/fcell.2017.00034.
Schmidt, T. H., Homsi, Y., & Lang, T. (2016). Oligomerization of the tetraspanin CD81 via the flexibility of its δ-loop. Biophysical Journal, 110, 2463–2474. https://doi.org/10.1016/j.bpj.2016.05.003.
Yue, S., Zhao, K., Erb, U., Rana, S., & Zöller, M. (2017). Joint features and complementarities of Tspan8 and CD151 revealed in knockdown and knockout models. Biochemical Society Transactions, 45, 437–447. https://doi.org/10.1042/BST20160298.
Park, C. S., Kim, T. K., Kim, H. G., Kim, Y. J., Jeoung, M. H., Lee, W. R., et al. (2016). Therapeutic targeting of tetraspanin8 in epithelial ovarian cancer invasion and metastasis. Oncogene, 35, 4540–4548. https://doi.org/10.1038/onc.2015.520.
Fang, T., Lin, J., Wang, Y., Chen, G., Huang, J., Chen, J., et al. (2016). Tetraspanin-8 promotes hepatocellular carcinoma metastasis by increasing ADAM12m expression. Oncotarget, 7, 40630–40643. doi: 10.18632/oncotarget.9769.
Wie, L., Li, Y., & Suo, Z. (2015). TSPAN8 promotes gastric cancer growth and metastasis via ERK MAPK pathway. International Journal of Clinical and Experimental Medicine, 8(6), 8599–8607.
El Kharbili, M., Robert, C., Witkowski, T., Danty-Berger, E., Barbollat-Boutrand, L., Masse, I., et al. (2017). Tetraspanin 8 is a novel regulator of ILK-driven β1 integrin adhesion and signaling in invasive melanoma cells. Oncotarget, 8(10), 17140–17155. https://doi.org/10.18632/oncotarget.15084.
Pan, S. J., Wu, Y. B., Cai, S., Pan, Y. X., Liu, W., Bian, L. G., et al. (2015). Over-expression of tetraspanin 8 in malignant glioma regulates tumor cell progression. Biochemical and Biophysical Research Communications, 458, 476–482. https://doi.org/10.1016/j.bbrc.2015.01.128.
Wang, H., Rana, S., Giese, N., Büchler, M. W., & Zöller, M. (2013). Tspan8, CD44v6 and alpha6beta4 are biomarkers of migrating pancreatic cancer-initiating cells. International Journal of Cancer, 133(2), 416–426. https://doi.org/10.1002/ijc.28044.
Madhavan, B., Yue, S., Galli, U., Rana, S., Groß, W., Müller, M., et al. (2015). Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. International Journal of Cancer, 136(11), 2616–2627. https://doi.org/10.1002/ijc.29324.
Greco, C., Bralet, M. P., Ailane, N., Dubart-Kupperschmitt, A., Rubinstein, E., Le Naour, F., et al. (2010). E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Research, 70(19), 7674–7683. https://doi.org/10.1158/0008-5472.CAN-09-4482.
Ailane, N., Greco, C., Zhu, Y., Sala-Valdés, M., Billard, M., Casal, I., et al. (2014). Effect of an anti-human Co-029/tspan8 mouse monoclonal antibody on tumor growth in a nude mouse model. Frontiers in Physiology, 5, 364. https://doi.org/10.3389/fphys.2014.00364.
Pan, S. J., Zhan, S. K., Pan, Y. X., Liu, W., Bian, L. G., Sun, B., et al. (2015). Tetraspanin 8-rictor-integrin α3 complex is required for glioma cell migration. International Journal of Molecular Sciences, 16, 5363–5374. https://doi.org/10.3390/ijms16035363.
Wang, Z., von Au, A., Schnölzer, M., Hackert, T., & Zöller, M. (2016). CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget, 7(34), 55409–55436. https://doi.org/10.18632/oncotarget.10580.
Yue, S., Mu, W., & Zöller, M. (2013). Tspan8 and CD151 promote metastasis by distinct mechanisms. European Journal of Cancer, 49(13), 2934–2948. https://doi.org/10.1016/j.ejca.2013.03.032.
Schmidt, F., Müller, M., Prox, J., Arnold, P., Schönherr, C., Tredup, C., et al. (2016). Tetraspanin 8 is an interactor of the metalloprotease meprin β within tetraspanin-enriched microdomains. Biological Chemistry, 397(9), 857–869. https://doi.org/10.1515/hsz-2016-0126.
Zhu, Y., Ailane, N., Sala-Valdés, M., Haghighi-Rad, F., Billard, M., Nguyen, V., et al. (2017). Multi-factorial modulation of colorectal carcinoma cells motility—partial coordination by the tetraspanin Co-029/tspan8. Oncotarget, 8(16), 27454–27470. https://doi.org/10.18632/oncotarget.16247.
Gesierich, S., Berezovskiy, I., Ryschich, E., & Zöller, M. (2006). Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Research, 66, 7083–7094.
Nazarenko, I., Rana, S., Baumann, A., McAlear, J., Hellwig, A., Trendelenburg, M., et al. (2010). Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Research, 70(4), 1668–1678. https://doi.org/10.1158/0008-5472.CAN-09-2470.
Rana, S., Claas, C., Kretz, C. C., Nazarenko, I., & Zöller, M. (2011). Activation-induced internalization differs for the tetraspanins CD9 and Tspan8: impact on tumor cell motility. The International Journal of Biochemistry & Cell Biology, 43(1), 106–119. https://doi.org/10.1016/j.biocel.2010.10.002.
Litvinov, S. V., Velders, M. P., Bakker, H. A., Fleuren, G. J., & Warnaar, S. O. (1994). Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. The Journal of Cell Biology, 125(2), 437–446.
Patriarca, C., Macchi, R. M., Marschner, A. K., & Mellstedt, H. (2012). Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treatment Reviews, 38(1), 68–75. https://doi.org/10.1016/j.ctrv.2011.04.002.
Imrich, S., Hachmeister, M., & Gires, O. (2012). EpCAM and its potential role in tumor-initiating cells. Cell Adhesion & Migration, 6, 30–38.
Maghzal, N., Vogt, E., Reintsch, W., Fraser, J. S., & Fagotto, F. (2010). The tumor-associated EpCAM regulates morphogenetic movements through intracellular signaling. The Journal of Cell Biology, 191, 645–659.
Maetzel, D., Denzel, S., Mack, B., Eggert, C., Bärr, G., & Gires, O. (2009). Nuclear signalling by tumour-associated antigen EpCAM. Nature Cell Biology, 11, 162–171.
Lin, C. W., Liao, M. Y., Lin, W. W., Wang, Y. P., Lu, T. Y., & Wu, H. C. (2012). Epithelial cell adhesion molecule regulates tumor initiation and tumorigenesis via activating reprogramming factors and epithelial-mesenchymal transition genes expression in colon cancer. The Journal of Biological Chemistry, 287, 39449–39459.
Wang, H., Stoecklein, N. H., Lin, P. P., & Gires, O. (2017). Circulating and disseminated tumor cells: diagnostic tools and therapeutic targets in motion. Oncotarget, 8(1), 1884–1912. https://doi.org/10.18632/oncotarget.12242.
Herreros-Pomares, A., Aguilar-Gallardo, C., Calabuig-Fariñas, S., Sirera, R., Jantus-Lewintre, E., & Camps, C. (2018). EpCAM duality becomes this molecule in a new Dr. Jekyll and Mr. Hyde tale. Critical Reviews in Oncology/Hematology, 126, 52–63. https://doi.org/10.1016/j.critrevonc.2018.03.006.
Biddle, A., Liang, X., Gammon, L., Fazil, B., Harper, L. J., Emich, H., et al. (2011). Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Research, 71(15), 5317–5326. https://doi.org/10.1158/0008-5472.CAN-11-1059.
Gires, O., Klein, C. A., & Baeuerle, P. A. (2009). On the abundance of EpCAM on cancer stem cells. Nature Reviews. Cancer, 9(2), 143; author reply 143. https://doi.org/10.1038/nrc2499-c1.
González, B., Denzel, S., Mack, B., Conrad, M., & Gires, O. (2009). EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem Cells, 27(8), 1782–1791. https://doi.org/10.1002/stem.97.
Lu, T. Y., Lu, R. M., Liao, M. Y., Yu, J., Chung, C. H., Kao, C. F., et al. (2010). Epithelial cell adhesion molecule regulation is associated with the maintenance of the undifferentiated phenotype of human embryonic stem cells. The Journal of Biological Chemistry, 285(12), 8719–8732. https://doi.org/10.1074/jbc.M109.077081.
Tamura, A., & Tsukita, S. (2014). Paracellular barrier and channel functions of TJ claudins in organizing biological systems: advances in the field of barriology revealed in knockout mice. Seminars in Cell & Developmental Biology, 36, 177–185. https://doi.org/10.1016/j.semcdb.2014.09.019.
Van Itallie, C. M., & Anderson, J. M. (2014). Architecture of tight junctions and principles of molecular composition. Seminars in Cell & Developmental Biology, 36, 157–165. https://doi.org/10.1016/j.semcdb.2014.08.011.
Ding, L., Lu, Z., Foreman, O., Tatum, R., Lu, Q., Renegar, R., et al. (2012). Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology, 142, 305–315. https://doi.org/10.1053/j.gastro.2011.
Tanaka, H., Takechi, M., Kiyonari, H., Shioi, G., Tamura, A., & Tsukita, S. (2015). Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut, 64, 1529–1538. https://doi.org/10.1136/gutjnl-2014-308419.
Lal-Nag, M., & Morin, P. J. (2009). The claudins. Genome Biology, 10, 235.
Van Itallie, C. M., & Anderson, J. M. (2013). Claudin interactions in and out of the tight junction. Tissue Barriers, 1, e25247.
Sjö, A., Magnusson, K. E., & Peterson, K. H. (2010). Protein kinase C activation has distinct effects on the localization, phosphorylation and detergent solubility of the claudin protein family in tight and leaky epithelial cells. The Journal of Membrane Biology, 236, 181–189.
Su, L., Nalle, S. C., Shen, L., Turner, E. S., Singh, G., Breskin, L. A., et al. (2013). TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology, 145(2), 407–415. https://doi.org/10.1053/j.gastro.2013.04.011.
Shen, L. (2012). Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Annals of the New York Academy of Sciences, 1258, 9–12518.
Stamatovic, S. M., Keep, R. F., & Andjelkovic, A. V. (2011). Tracing the endocytosis of claudin-5 in brain endothelial cells. Methods in Molecular Biology, 762, 303–320. https://doi.org/10.1007/978-1-61779-185-7_22.
Heiler, S., Mu, W., Zöller, M., & Thuma, F. (2015). The importance of claudin-7 palmitoylation on membrane subdomain localization and metastasis-promoting activities. Cell Communication and Signaling: CCS, 13, 29.
Thuma, F., Heiler, S., Schnölzer, M., & Zöller, M. (2016). Palmitoylated claudin7 captured in glycolipid-enriched membrane microdomains promotes metastasis via associated transmembrane and cytosolic molecules. Oncotarget, 7, 30659–30677. https://doi.org/10.18632/oncotarget.8928.
Rao, Y., Rückert, C., Saenger, W., & Haucke, V. (2012). The early steps of endocytosis: from cargo selection to membrane deformation. European Journal of Cell Biology, 91, 226–233. https://doi.org/10.1016/j.ejcb.2011.02.004.
Tauro, B. J., Greening, D. W., Mathias, R. A., Mathivanan, S., Ji, H., & Simpson, R. J. (2013). Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Molecular & Cellular Proteomics, 12, 587–598.
Deshmukh, A., Binju, M., Arfuso, F., Newsholme, P., & Dharmarajan, A. (2017). Role of epigenetic modulation in cancer stem cell fate. The International Journal of Biochemistry & Cell Biology, 90, 9–16. https://doi.org/10.1016/j.biocel.2017.07.003.
Godoy, P., Schmidt-Heck, W., Hellwig, B., Nell, P., Feuerborn, D., Rahnenführer, J., et al. (2018). Assessment of stem cell differentiation based on genome-wide expression profiles. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 373(1750), 20170221. https://doi.org/10.1098/rstb.2017.0221.
Niwa, H. (2018). The principles that govern transcription factor network functions in stem cells. Development, 145(6), 157420. https://doi.org/10.1242/dev.157420.
Herreros-Villanueva, M., Bujanda, L., Billadeau, D. D., & Zhang, J. S. (2014). Embryonic stem cell factors and pancreatic cancer. World Journal of Gastroenterology, 20(9), 2247–2254. https://doi.org/10.3748/wjg.v20.i9.2247.
Herreros-Villanueva, M., Zhang, J. S., Koenig, A., Abel, E. V., Smyrk, T. C., Bamlet, W. R., et al. (2013). SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis, 2, e61. https://doi.org/10.1038/oncsis.2013.23.
Rhim, A. D., Mirek, E. T., Aiello, N. M., Maitra, A., Bailey, J. M., McAllister, F., et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148(1–2), 349–361. https://doi.org/10.1016/j.cell.2011.11.025.
Wang, S., Huang, S., & Sun, Y. L. (2017). Epithelial-mesenchymal transition in pancreatic cancer: a review. BioMed Research International, 2017, 2646148. https://doi.org/10.1155/2017/2646148.
Roe, J. S., Hwang, C. I., Somerville, T. D. D., Milazzo, J. P., Lee, E. J., Da Silva, B., et al. (2017). Enhancer reprogramming promotes pancreatic cancer metastasis. Cell, 170(5), 875–888.e20. https://doi.org/10.1016/j.cell.2017.07.007.
Kreso, A., & Dick, J. E. (2014). Evolution of the cancer stem cell model. Cell Stem Cell, 14(3), 275–291. https://doi.org/10.1016/j.stem.2014.02.006.
Katoh, M. (2017). Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (review). International Journal of Oncology, 51(5), 1357–1369. https://doi.org/10.3892/ijo.2017.4129.
Fearon, E. R., & Wicha, M. S. (2014). KRAS and cancer stem cells in APC-mutant colorectal cancer. Journal of the National Cancer Institute, 106(2), djt444. https://doi.org/10.1093/jnci/djt444.
Zhang, F., Sun, H., Zhang, S., Yang, X., Zhang, G., & Su, T. (2017). Overexpression of PER3 inhibits self-renewal capability and chemoresistance of colorectal cancer stem-like cells via inhibition of notch and β-catenin signaling. Oncology Research, 25(5), 709–719. https://doi.org/10.3727/096504016X14772331883976.
Batsaikhan, B. E., Yoshikawa, K., Kurita, N., Iwata, T., Takasu, C., Kashihara, H., et al. (2014). Cyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cells. Anticancer Research, 34(11), 6339–6344.
Whissell, G., Montagni, E., Martinelli, P., Hernando-Momblona, X., Sevillano, M., Jung, P., et al. (2014). The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nature Cell Biology, 16(7), 695–707. https://doi.org/10.1038/ncb2992.
Chen, J., Shao, R., Li, F., Monteiro, M., Liu, J. P., Xu, Z. P., et al. (2015). PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clinical and Experimental Pharmacology & Physiology, 42(12), 1317–1326. https://doi.org/10.1111/1440-1681.12493.
Pelicci, P. G., Dalton, P., & Giorgio, M. (2013). The other face of ROS: a driver of stem cell expansion in colorectal cancer. Cell Stem Cell, 12(6), 635–636. https://doi.org/10.1016/j.stem.2013.05.023.
Hong, A. W., Meng, Z., & Guan, K. L. (2016). The Hippo pathway in intestinal regeneration and disease. Nature Reviews. Gastroenterology & Hepatology, 13(6), 324–337. https://doi.org/10.1038/nrgastro.2016.59.
Sikandar, S. S., Pate, K. T., Anderson, S., Dizon, D., Edwards, R. A., Waterman, M. L., et al. (2010). NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Research, 70(4), 1469–1478. https://doi.org/10.1158/0008-5472.CAN-09-2557.
Apostolou, P., Toloudi, M., Ioannou, E., Kourtidou, E., Chatziioannou, M., Kopic, A., et al. (2013). Study of the interaction among Notch pathway receptors, correlation with stemness, as well as their interaction with CD44, dipeptidyl peptidase-IV, hepatocyte growth factor receptor and the SETMAR transferase, in colon cancer stem cells. Journal of Receptor and Signal Transduction Research, 33(6), 353–358. https://doi.org/10.3109/10799893.2013.828072.
Fender, A. W., Nutter, J. M., Fitzgerald, T. L., Bertrand, F. E., & Sigounas, G. (2015). Notch-1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. Journal of Cellular Biochemistry, 116(11), 2517–2527. https://doi.org/10.1002/jcb.25196.
Jin, L., Vu, T., Yuan, G., & Datta, P. K. (2017). STRAP promotes stemness of human colorectal cancer via epigenetic regulation of the NOTCH pathway. Cancer Research, 77(20), 5464–5478. https://doi.org/10.1158/0008-5472.CAN-17-0286.
Voorneveld, P. W., Kodach, L. L., Jacobs, R. J., van Noesel, C. J., Peppelenbosch, M. P., Korkmaz, K. S., et al. (2015). The BMP pathway either enhances or inhibits the Wnt pathway depending on the SMAD4 and p53 status in CRC. British Journal of Cancer, 112(1), 122–130. https://doi.org/10.1038/bjc.2014.560.
Kim, B. R., Oh, S. C., Lee, D. H., Kim, J. L., Lee, S. Y., Kang, M. H., et al. (2015). BMP-2 induces motility and invasiveness by promoting colon cancer stemness through STAT3 activation. Tumour Biology, 36(12), 9475–9486. https://doi.org/10.1007/s13277-015-3681-y.
Catalano, V., Dentice, M., Ambrosio, R., Luongo, C., Carollo, R., Benfante, A., et al. (2016). Activated thyroid hormone promotes differentiation and chemotherapeutic sensitization of colorectal cancer stem cells by regulating Wnt and BMP4 signaling. Cancer Research, 76(5), 1237–1244. https://doi.org/10.1158/0008-5472.CAN-15-1542.
Xue, R., Jia, K., Wang, J., Yang, L., Wang, Y., Gao, L., et al. (2018). A rising star in pancreatic diseases: pancreatic stellate cells. Frontiers in Physiology, 9, 754. https://doi.org/10.3389/fphys.2018.00754.
Zeuner, A., Todaro, M., Stassi, G., & De Maria, R. (2014). Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell, 15(6), 692–705. https://doi.org/10.1016/j.stem.2014.11.012.
Calon, A., Tauriello, D. V., & Batlle, E. (2014). TGF-beta in CAF-mediated tumor growth and metastasis. Seminars in Cancer Biology, 25, 15–22. https://doi.org/10.1016/j.semcancer.2013.12.008.136.
Wang, K., & Karin, M. (2015). Tumor-elicited inflammation and colorectal cancer. Advances in Cancer Research, 128, 173–196. https://doi.org/10.1016/bs.acr.2015.04.014.
Lotti, F., Jarrar, A. M., Pai, R. K., Hitomi, M., Lathia, J., Mace, A., et al. (2013). Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. The Journal of Experimental Medicine, 210(13), 2851–2872. https://doi.org/10.1084/jem.20131195.
Koukourakis, M. I., Giatromanolaki, A., Harris, A. L., & Sivridis, E. (2006). Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Research, 66(2), 632–637.
Koliaraki, V., Pallangyo, C. K., Greten, F. R., & Kollias, G. (2017). Mesenchymal cells in colon cancer. Gastroenterology, 152(5), 964–979. https://doi.org/10.1053/j.gastro.2016.11.049.
Lu, J., Ye, X., Fan, F., Xia, L., Bhattacharya, R., Bellister, S., et al. (2013). Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell, 23(2), 171–185. https://doi.org/10.1016/j.ccr.2012.12.021.
Roberts, K. J., Kershner, A. M., & Beachy, P. A. (2017). The stromal niche for epithelial stem cells: a template for regeneration and a brake on malignancy. Cancer Cell, 32(4), 404–410. https://doi.org/10.1016/j.ccell.2017.08.007.
Gerling, M., Büller, N. V., Kirn, L. M., Joost, S., Frings, O., Englert, B., et al. (2016). Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nature Communications, 7, 12321. https://doi.org/10.1038/ncomms12321.
Nicolas, F. E. (2017). Role of ncRNAs in development, diagnosis and treatment of human cancer. Recent Patents on Anti-Cancer Drug Discovery, 12(2), 128–135. https://doi.org/10.2174/1574892812666170105113415.
Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297.
Basyuk, E., Suavet, F., Doglio, A., Bordonné, R., & Bertrand, E. (2003). Human let-7 stem-loop precursors harbor features of RNase III cleavage products. Nucleic Acids Research, 31(22), 6593–6597.
Lee, Y., Ahn, C., Han, J., Choi, H., Kim, J., Yim, J., et al. (2003). The nuclear RNase III Drosha initiates microRNA processing. Nature, 425(6956), 415–419.
Chendrimada, T. P., Gregory, R. I., Kumaraswamy, E., Norman, J., Cooch, N., Nishikura, K., et al. (2005). TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature, 436(7051), 740–744.
Denli, A. M., Tops, B. B., Plasterk, R. H., Ketting, R. F., & Hannon, G. J. (2004). Processing of primary microRNAs by the microprocessor complex. Nature, 432(7014), 231–235.
Seok, H., Ham, J., Jang, E. S., & Chi, S. W. (2016). MicroRNA target recognition: insights from transcriptome-wide non-canonical interactions. Molecules and Cells, 39(5), 375–381. https://doi.org/10.14348/molcells.2016.0013.
Nicoloso, M. S., Spizzo, R., Shimizu, M., Rossi, S., & Calin, G. A. (2009). MicroRNAs—the micro steering wheel of tumour metastases. Nature Reviews. Cancer, 9(4), 293–302. https://doi.org/10.1038/nrc2619.
Acunzo, M., Romano, G., Wernicke, D., & Croce, C. M. (2015). MicroRNA and cancer—a brief overview. Adv Biol Regul, 57, 1–9. https://doi.org/10.1016/j.jbior.2014.09.013.
Giovannetti, E., van der Borden, C. L., Frampton, A. E., Ali, A., Firuzi, O., & Peters, G. J. (2017). Never let it go: stopping key mechanisms underlying metastasis to fight pancreatic cancer. Seminars in Cancer Biology, 44, 43–59. https://doi.org/10.1016/j.semcancer.2017.04.006.
Liu, X., Fu, Q., Du, Y., Yang, Y., & Cho, W. C. (2016). MicroRNA as regulators of cancer stem cells and chemoresistance in colorectal cancer. Current Cancer Drug Targets, 16(9), 738–754.
Mamoori, A., Gopalan, V., Smith, R. A., & Lam, A. K. (2016). Modulatory roles of microRNAs in the regulation of different signalling pathways in large bowel cancer stem cells. Biology of the Cell, 108(3), 51–64. https://doi.org/10.1111/boc.201500062.
Kung, J. T., Colognori, D., & Lee, J. T. (2013). Long noncoding RNAs: past, present, and future. Genetics, 193(3), 651–669. https://doi.org/10.1534/genetics.112.146704.
Poliseno, L., Salmena, L., Zhang, J., Carver, B., Haveman, W. J., & Pandolfi, P. P. (2010). A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature, 465(7301), 1033–1038. https://doi.org/10.1038/nature09144.
Johnsson, P., Ackley, A., Vidarsdottir, L., Lui, W. O., Corcoran, M., Grandér, D., et al. (2013). A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nature Structural & Molecular Biology, 20(4), 440–446. https://doi.org/10.1038/nsmb.2516.
Li, T., Mo, X., Fu, L., Xiao, B., & Guo, J. (2016). Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget, 7(8), 8601–8612. https://doi.org/10.18632/oncotarget.6926.
Sanchez-Mejias, A., & Tay, Y. (2015). Competing endogenous RNA networks: tying the essential knots for cancer biology and therapeutics. Journal of Hematology & Oncology, 8, 30. https://doi.org/10.1186/s13045-015-0129-1.
Ebert, M. S., Neilson, J. R., & Sharp, P. A. (2007). MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature Methods, 4(9), 721–726.
Kopp, F., & Mendell, J. T. (2018). Functional classification and experimental dissection of long noncoding RNAs. Cell, 172(3), 393–407. https://doi.org/10.1016/j.cell.2018.01.011.
Rinn, J. L., Kertesz, M., Wang, J. K., Squazzo, S. L., Xu, X., Brugmann, S. A., et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell, 129(7), 1311–1323.
Hung, T., Wang, Y., Lin, M. F., Koegel, A. K., Kotake, Y., Grant, G. D., et al. (2011). Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature Genetics, 43(7), 621–629. https://doi.org/10.1038/ng.848.
Liu, C., Wu, H. T., Zhu, N., Shi, Y. N., Liu, Z., Ao, B. X., et al. (2016). Steroid receptor RNA activator: biologic function and role in disease. Clinica Chimica Acta, 459, 137–146. https://doi.org/10.1016/j.cca.2016.06.004.
Thomson, D. W., & Dinger, M. E. (2016). Endogenous microRNA sponges: evidence and controversy. Nature Reviews. Genetics, 17(5), 272–283. https://doi.org/10.1038/nrg.2016.20.
Dykes, I. M., & Emanueli, C. (2017). Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genomics, Proteomics & Bioinformatics, 15(3), 177–186. https://doi.org/10.1016/j.gpb.2016.12.005.
Deng, H., Wang, J. M., Li, M., Tang, R., Tang, K., Su, Y., et al. (2017). Long non-coding RNAs: new biomarkers for prognosis and diagnosis of colon cancer. Tumour Biology, 39(6), 1010428317706332. https://doi.org/10.1177/1010428317706332.
Yang, Y., Junjie, P., Sanjun, C., & Ma, Y. (2017). Long non-coding RNAs in colorectal cancer: progression and future directions. Journal of Cancer, 8(16), 3212–3225. https://doi.org/10.7150/jca.19794.
Yang, S., Sun, Z., Zhou, Q., Wang, W., Wang, G., Song, J., et al. (2018). MicroRNAs, long noncoding RNAs, and circular RNAs: potential tumor biomarkers and targets for colorectal cancer. Cancer Management and Research, 10, 2249–2257. https://doi.org/10.2147/CMAR.S166308.
Han, T., Hu, H., Zhuo, M., Wang, L., Cui, J. J., Jiao, F., et al. (2016). Long non-coding RNA: an emerging paradigm of pancreatic cancer. Current Molecular Medicine, 16(8), 702–709.
Duguang, L., Jin, H., Xiaowei, Q., Peng, X., Xiaodong, W., Zhennan, L., et al. (2017). The involvement of lncRNAs in the development and progression of pancreatic cancer. Cancer Biology & Therapy, 18(12), 927–936. https://doi.org/10.1080/15384047.2017.1385682.
Huang, X., Xiao, R., Pan, S., Yang, X., Yuan, W., Tu, Z., et al. (2017). Uncovering the roles of long non-coding RNAs in cancer stem cells. Journal of Hematology & Oncology, 10(1), 62. https://doi.org/10.1186/s13045-017-0428-9.
Heery, R., Finn, S. P., Cuffe, S., & Gray, S. G. (2017). Long non-coding RNAs: key regulators of epithelial-mesenchymal transition, tumour drug resistance and cancer stem cells. Cancers (Basel), 9(4), E38. https://doi.org/10.3390/cancers9040038.
Chi, H. C., Tsai, C. Y., Tsai, M. M., Yeh, C. T., & Lin, K. H. (2017). Roles of long noncoding RNAs in recurrence and metastasis of radiotherapy-resistant cancer stem cells. International Journal of Molecular Sciences, 18(9), E1903. https://doi.org/10.3390/ijms18091903.
Théry, C., Zitvogel, L., & Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nature Reviews. Immunology, 2(8), 569–579. https://doi.org/10.1038/nri855.
Boukouris, S., & Mathivanan, S. (2015). Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics. Clinical Applications, 9, 358–367. https://doi.org/10.1002/prca.201400114.
Jia, S., Zocco, D., Samuels, M. L., Chou, M. F., Chammas, R., Skog, J., et al. (2014). Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Review of Molecular Diagnostics, 14, 307–321. https://doi.org/10.1586/14737159.2014.893828.
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9, 654–659.
Lo Cicero, A., Stahl, P. D., & Raposo, G. (2015). Extracellular vesicles shuffling intercellular messages: for good or for bad. Current Opinion in Cell Biology, 35, 69–77. https://doi.org/10.1016/j.ceb.2015.04.013.
Javeed, N., & Mukhopadhyay, D. (2017). Exosomes and their role in the micro-/macro-environment: a comprehensive review. Journal of Biomedical Research, 31(5), 386–394. https://doi.org/10.7555/JBR.30.20150162.
Burrello, J., Monticone, S., Gai, C., Gomez, Y., Kholia, S., & Camussi, G. (2016). Stem cell-derived extracellular vesicles and immune-modulation. Frontiers in Cell and Development Biology, 4, 83. https://doi.org/10.3389/fcell.2016.00083.
Todorova, D., Simoncini, S., Lacroix, R., Sabatier, F., & Dignat-George, F. (2017). Extracellular vesicles in angiogenesis. Circulation Research, 120(10), 1658–1673. https://doi.org/10.1161/CIRCRESAHA.117.309681.
Rajagopal, C., & Harikumar, K. B. (2018). The origin and functions of exosomes in cancer. Frontiers in Oncology, 8, 66. https://doi.org/10.3389/fonc.2018.00066.
Yang, B., Chen, Y., & Shi, J. (2018). Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Advanced Materials, e1802896. https://doi.org/10.1002/adma.201802896.
Colombo, M., Raposo, G., & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326.
van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews. Molecular Cell Biology, 19(4), 213–228. https://doi.org/10.1038/nrm.2017.125.
Abels, E. R., & Breakefield, X. O. (2016). Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cellular and Molecular Neurobiology, 36, 301–312. https://doi.org/10.1007/s10571-016-0366-z.
Villarroya-Beltri, C., Baixauli, F., Gutiérrez-Vázquez, C., Sánchez-Madrid, F., & Mittelbrunn, M. (2014). Sorting it out: regulation of exosome loading. Seminars in Cancer Biology, 28, 3–13. https://doi.org/10.1016/j.semcancer.2014.04.009.
Choi, D. S., Kim, D. K., Kim, Y. K., & Gho, Y. S. (2015). Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrometry Reviews, 34, 474–490. https://doi.org/10.1002/pmic.201200329.
Nabhan, J. F., Hu, R., Oh, R. S., Cohen, S. N., & Lu, Q. (2012). Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences of the United States of America, 109, 4146–4151. https://doi.org/10.1073/pnas.1200448109.
Egea-Jimenez, A. L., & Zimmermann, P. (2018). Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. Journal of Lipid Research, jlr.R083964. https://doi.org/10.1194/jlr.R083964.
Kajimoto, T., Okada, T., Miya, S., Zhang, L., & Nakamura, S. (2013). Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multi-vesicular endosomes. Nature Communications, 4, 2712. https://doi.org/10.1038/ncomms3712.
Shen, B., Fang, Y., Wu, N., & Gould, S. J. (2011). Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. The Journal of Biological Chemistry, 286, 44162–44176. https://doi.org/10.1074/jbc.M111.274803.
Guo, B. B., Bellingham, S. A., & Hill, A. F. (2015). The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. The Journal of Biological Chemistry, 290, 3455–3467. https://doi.org/10.1074/jbc.M115.684258.
Vedeler, A., Hollås, H., Grindheim, A. K., & Raddum, A. M. (2012). Multiple roles of annexin A2 in post-transcriptional regulation of gene expression. Current Protein & Peptide Science, 13, 401–412.
Janas, T., Janas, M. M., Sapoń, K., & Janas, T. (2015). Mechanisms of RNA loading into exosomes. FEBS Letters, 589(13), 1391–1398. https://doi.org/10.1016/j.febslet.2015.04.036.
Villarroya-Beltri, C., Gutierrez-Vazquez, C., Sanchez-Cabo, F., Pérez-Hernández, D., Vázquez, J., Martin-Cofreces, N., et al. (2013). Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nature Communications, 4, 2980. https://doi.org/10.1038/ncomms3980.
Gezer, U., Özgür, E., Cetinkaya, M., Isin, M., & Dalay, N. (2014). Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biology International, 38(9), 1076–1079. https://doi.org/10.1002/cbin.10301.
Hessvik, N. P., & Llorente, A. (2018). Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Sciences, 75(2), 193–208. https://doi.org/10.1007/s00018-017-2595-9.
Ji, H., Greening, D. W., Barnes, T. W., Lim, J. W., Tauro, B. J., Rai, A., et al. (2013). Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics, 13, 1672–1686. https://doi.org/10.1002/pmic.201200562.
Kowal, J., Arras, G., Colombo, M., Jouve, M., Morath, J. P., Primdal-Bengtson, B., et al. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America, 113, E968–E977. https://doi.org/10.1073/pnas.1521230113.
Subra, C., Grand, D., Laulagnier, K., Stella, A., Lambeau, G., Paillasse, M., et al. (2010). Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. Journal of Lipid Research, 51, 2105–2120.
Skotland, T., Hessvik, N. P., Sandvig, K., & Llorente, A. (2018). Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. Journal of Lipid Research, jlr.R084343. https://doi.org/10.1194/jlr.R084343.
Sharma, R., Huang, X., Brekken, R. A., & Schroit, A. J. (2017). Detection of phosphatidylserine-positive exosomes for the diagnosis of early-stage malignancies. British Journal of Cancer, 117, 545–552. https://doi.org/10.1038/bjc.2017.183.
Lesur, A., & Domon, B. (2015). Advances in high-resolution accurate mass spectrometry application to targeted proteomics. Proteomics, 15, 880–890. https://doi.org/10.1002/pmic.201400450.
Schey, K. L., Luther, J. M., & Rose, K. L. (2015). Proteomics characterization of exosome cargo. Methods, 87, 75–82. https://doi.org/10.1016/j.ymeth.2015.03.018.
Zöller, M. (2009). Tetraspanins: push and pull in suppressing and promoting metastasis. Nature Reviews. Cancer, 9, 40–55. https://doi.org/10.1038/nrc2543.
Mathivanan, S., Ji, H., & Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics, 73, 1907–1920. https://doi.org/10.1016/j.jprot.2010.06.006.
Greening, D. W., Xu, R., Gopal, S. K., Rai, A., & Simpson, R. J. (2017). Proteomic insights into extracellular vesicle biology—defining exosomes and shed microvesicles. Expert Review of Proteomics, 14(1), 69–95. https://doi.org/10.1080/14789450.2017.1260450.
Rosa-Fernandes, L., Rocha, V. B., Carregari, V. C., Urbani, A., & Palmisano, G. (2017). A perspective on extracellular vesicles proteomics. Frontiers in Chemistry, 5, 102. https://doi.org/10.3389/fchem.2017.00102.
Mears, R., Craven, R. A., Hanrahan, S., Totty, N., Upton, C., Young, S. L., et al. (2004). Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics, 4(12), 4019–4031.
Al-Nedawi, K., Meehan, B., Micallef, J., Lhotak, V., May, L., Guha, A., et al. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature Cell Biology, 10, 619–624. https://doi.org/10.1038/ncb1725.
Osteikoetxea, X., Benke, M., Rodriguez, M., Pálóczi, K., Sódar, B. W., Szvicsek, Z., et al. (2018). Detection and proteomic characterization of extracellular vesicles in human pancreatic juice. Biochemical and Biophysical Research Communications, 499(1), 37–43. https://doi.org/10.1016/j.bbrc.2018.03.107.
Marhaba, R., Klingbeil, P., Nuebel, T., Nazarenko, I., Buechler, M. W., & Zöller, M. (2008). CD44 and EpCAM: cancer-initiating cell markers. Current Molecular Medicine, 8(8), 784–804.
Matsumoto, K., Umitsu, M., De Silva, D. M., Roy, A., & Bottaro, D. P. (2017). Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Science, 108(3), 296–307. https://doi.org/10.1111/cas.13156.
Demory Beckler, M., Higginbotham, J. N., Franklin, J. L., Ham, A. J., Halvey, P. J., Imasuen, I. E., et al. (2013). Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Molecular & Cellular Proteomics, 12, 343–355. https://doi.org/10.1074/mcp.M112.022806.
Jung, T., Castellana, D., Klingbeil, P., Cuesta Hernández, I., Vitacolonna, M., Orlicky, D. J., et al. (2009). CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia, 11(10), 1093–1105.
Anami, K., Oue, N., Noguchi, T., Sakamoto, N., Sentani, K., Hayashi, T., et al. (2016). TSPAN8, identified by Escherichia coli ampicillin secretion trap, is associated with cell growth and invasion in gastric cancer. Gastric Cancer, 19(2), 370–380. https://doi.org/10.1007/s10120-015-0478-z.
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527(7578), 329–335. https://doi.org/10.1038/nature15756.
Paolillo, M., & Schinelli, S. (2017). Integrins and exosomes, a dangerous liaison in cancer progression. Cancers (Basel), 9(8), E95. https://doi.org/10.3390/cancers9080095.
Philip, R., Heiler, S., Mu, W., Büchler, M. W., Zöller, M., & Thuma, F. (2015). Claudin-7 promotes the epithelial-mesenchymal transition in human colorectal cancer. Oncotarget, 6(4), 2046–2063.
Marimpietri, D., Petretto, A., Raffaghello, L., Pezzolo, A., Gagliani, C., Tacchetti, C., et al. (2013). Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One, 8(9), e75054. https://doi.org/10.1371/journal.pone.0075054.
Rappa, G., Mercapide, J., Anzanello, F., Pope, R. M., & Lorico, A. (2013). Biochemical and biological characterization of exosomes containing prominin-1/CD133. Molecular Cancer, 12, 62. https://doi.org/10.1186/1476-4598-12-62.
Kumar, D., Gupta, D., Shankar, S., & Srivastava, R. K. (2015). Biomolecular characterization of exosomes released from cancer stem cells: possible implications for biomarker and treatment of cancer. Oncotarget, 10.18632/oncotarget.2462, 6, 3280, 3291.
Zöller, M. (2016). Exosomes in cancer disease. Methods in Molecular Biology, 1381, 111–149. https://doi.org/10.1007/978-1-4939-3204-7_7.
Mulcahy, L. A., Pink, R. C., & Carter, D. R. (2014). Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles, 3. https://doi.org/10.3402/jev.v3.24641.
Buzás, E. I., Tóth, E. Á., Sódar, B. W., & Szabó-Taylor, K. É. (2018). Molecular interactions at the surface of extracellular vesicles. Seminars in Immunopathology. https://doi.org/10.1007/s00281-018-0682-0.
Rackov, G., Garcia-Romero, N., Esteban-Rubio, S., Carrión-Navarro, J., Belda-Iniesta, C., & Ayuso-Sacido, A. (2018). Vesicle-mediated control of cell function: the role of extracellular matrix and microenvironment. Frontiers in Physiology, 9, 651. https://doi.org/10.3389/fphys.2018.00651.
Sanderson, R.D., Bandari, S.K., & Vlodavsky, I. (2017). Proteases and glycosidases on the surface of exosomes: newly discovered mechanisms for extracellular remodeling. Matrix Biol, S0945-053X(17)30311-30316. doi: https://doi.org/10.1016/j.matbio.2017.10.007.
Mu, W., Rana, S., & Zöller, M. (2013). Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia, 15, 875–887.
Wang, L., Hu, L., Zhou, X., Xiong, Z., Zhang, C., Shehada, H. M. A., et al. (2017). Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Scientific Reports, 7(1), 13321. https://doi.org/10.1038/s41598-017-12919-x.
Sung, B. H., Ketova, T., Hoshino, D., Zijlstra, A., & Weaver, A. M. (2015). Directional cell movement through tissues is controlled by exosome secretion. Nature Communications, 6, 7164. https://doi.org/10.1038/ncomms8164.
Del Vecchio, F., Lee, G. H., Hawezi, J., Bhome, R., Pugh, S., Sayan, E., et al. (2018). Long non-coding RNAs within the tumour microenvironment and their role in tumour-stroma cross-talk. Cancer Letters, 421, 94–102. https://doi.org/10.1016/j.canlet.2018.02.022.
French, K. C., Antonyak, M. A., & Cerione, R. A. (2017). Extracellular vesicle docking at the cellular port: extracellular vesicle binding and uptake. Seminars in Cell & Developmental Biology, 67, 48–55. https://doi.org/10.1016/j.semcdb.2017.01.002.
Moller-Tank, S., & Maury, W. (2014). Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology, 468-470, 565–580. https://doi.org/10.1016/j.virol.2014.09.009.
Christianson, H. C., Svensson, K. J., van Kuppevelt, T. H., Li, J. P., & Belting, M. (2013). Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proceedings of the National Academy of Sciences of the United States of America, 110, 17380–17385. https://doi.org/10.1073/pnas.1304266110.
Fei, F., Joo, E. J., Tarighat, S. S., Schiffer, I., Paz, H., Fabbri, M., et al. (2015). B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget, 6, 11378–11394. https://doi.org/10.18632/oncotarget.3409.
Gomes, J., Gomes-Alves, P., Carvalho, S. B., Peixoto, C., Alves, P. M., Altevogt, P., et al. (2015). Extracellular vesicles from ovarian carcinoma cells display specific glycosignatures. Biomolecules, 5, 1741–1761. https://doi.org/10.3390/biom5031741.
Rana, S., Yue, S., Stadel, D., & Zöller, M. (2012). Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. The International Journal of Biochemistry & Cell Biology, 44(9), 1574–1584. https://doi.org/10.1016/j.biocel.2012.06.018.
Montecalvo, A., Larregina, A. T., Shufesky, W. J., Stolz, D. B., Sullivan, M. L., Karlsson, J. M., et al. (2012). Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood, 119, 756–766. https://doi.org/10.1182/blood-2011-02-338004.
Del Conde, I., Shrimpton, C. N., Thiagarajan, P., & López, J. A. (2005). Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood, 106, 1604–1611. https://doi.org/10.1182/blood-2004-03-1095.
Tian, T., Zhu, Y. L., Hu, F. H., Wang, Y. Y., Huang, N. P., & Xiao, Z. D. (2013). Dynamics of exosome internalization and trafficking. Journal of Cellular Physiology, 228, 1487–1495. https://doi.org/10.1002/jcp.24304.
Feng, D., Zhao, W. L., Ye, Y. Y., Bai, X. C., Liu, R. Q., Chang, L. F., et al. (2010). Cellular internalization of exosomes occurs through phagocytosis. Traffic, 11, 675–687. https://doi.org/10.1111/j.1600-0854.2010.01041.x.
Freeman, S. A., & Grinstein, S. (2014). Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunological Reviews, 262, 193–215. https://doi.org/10.1111/imr.12212.
Nakase, I., Kobayashi, N. B., Takatani-Nakase, T., & Yoshida, T. (2015). Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Scientific Reports, 5, 10300. https://doi.org/10.1038/srep10300.
Thuma, F., & Zöller, M. (2014). Outsmart tumor exosomes to steal the cancer initiating cell its niche. Seminars in Cancer Biology, 28, 39–50. https://doi.org/10.1016/j.semcancer.2014.02.011.
Nanbo, A., Kawanishi, E., Yoshida, R., & Yoshiyama, H. (2013). Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. Journal of Virology, 87, 10334–10347. https://doi.org/10.1128/JVI.01310-13.
Lakkaraju, A., & Rodriguez-Boulan, E. (2008). Itinerant exosomes: emerging roles in cell and tissue polarity. Trends in Cell Biology, 18, 199–209. https://doi.org/10.1016/j.tcb.2008.03.002.
Leone, D. A., Peschel, A., Brown, M., Schachner, H., Ball, M. J., Gyuraszova, M., et al. (2017). Surface LAMP-2 is an endocytic receptor that diverts antigen internalized by human dendritic cells into highly immunogenic exosomes. Journal of Immunology, 199, 531–546. https://doi.org/10.4049/jimmunol.1601263.
Holder, B., Jones, T., Sancho Shimizu, V., Rice, T. F., Donaldson, B., Bouqueau, M., et al. (2016). Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic, 17, 168–178. https://doi.org/10.1111/tra.12352.
Heusermann, W., Hean, J., Trojer, D., Steib, E., von Bueren, S., Graff-Meyer, A., et al. (2016). Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. The Journal of Cell Biology, 213, 173–184. https://doi.org/10.1083/jcb.201506084.
Svensson, K. J., Kucharzewska, P., Christianson, H. C., Sköld, S., Löfstedt, T., Johansson, M. C., et al. (2011). Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13147–13152. https://doi.org/10.1073/pnas.1104261108.
Zhang, X., Wang, X., Zhu, H., Kranias, E. G., Tang, Y., Peng, T., et al. (2012). Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One, 7(3), e32765. https://doi.org/10.1371/journal.pone.0032765.
Arscott, W. T., Tandle, A. T., Zhao, S., Shabason, J. E., Gordon, I. K., Schlaff, C. D., et al. (2013). Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Translational Oncology, 6(6), 638–648.
Atay, S., Gercel-Taylor, C., & Taylor, D. D. (2011). Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1β production by macrophages. American Journal of Reproductive Immunology, 66(4), 259–269. https://doi.org/10.1111/j.1600-0897.2011.00995.x.
Fedele, C., Singh, A., Zerlanko, B. J., Iozzo, R. V., & Languino, L. R. (2015). The αvβ6 integrin is transferred intercellularly via exosomes. The Journal of Biological Chemistry, 290, 4545–4551. https://doi.org/10.1074/jbc.C114.617662.
Gu, X., Erb, U., Büchler, M. W., & Zöller, M. (2015). Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. International Journal of Cancer, 136, E74–E84. https://doi.org/10.1002/ijc.29100.
Lamichhane, T. N., Jeyaram, A., Patel, D. B., Parajuli, B., Livingston, N. K., Arumugasaamy, N., et al. (2016). Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cellular and Molecular Bioengineering, 9, 315–324. https://doi.org/10.1007/s12195-016-0457-4.
Saari, H., Lázaro-Ibáñez, E., Viitala, T., Vuorimaa-Laukkanen, E., Siljander, P., & Yliperttula, M. (2015). Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. Journal of Controlled Release, 220(Pt B), 727–737. https://doi.org/10.1016/j.jconrel.2015.09.031.
Kapustin, A. N., Schoppet, M., Schurgers, L. J., Reynolds, J. L., McNair, R., Heiss, A., et al. (2017). Prothrombin loading of vascular smooth muscle cell-derived exosomes regulates coagulation and calcification. Arteriosclerosis, Thrombosis, and Vascular Biology, 37, e22–e32. https://doi.org/10.1161/ATVBAHA.116.308886.
Zarovni, N., Corrado, A., Guazzi, P., Zocco, D., Lari, E., Radano, G., et al. (2015). Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods, 87, 46–58. https://doi.org/10.1016/j.ymeth.2015.05.028.
Théry, C., Duban, L., Segura, E., Véron, P., Lantz, O., & Amigorena, S. (2002). Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nature Immunology, 3, 1156–1162. https://doi.org/10.1038/ni854.
Simhadri, V. R., Reiners, K. S., Hansen, H. P., Topolar, D., Simhadri, V. L., Nohroudi, K., et al. (2008). Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One, 3, e3377. https://doi.org/10.1371/journal.pone.0003377.
Viaud, S., Terme, M., Flament, C., Taieb, J., André, F., Novault, S., et al. (2009). Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS, 4(3), e4942. https://doi.org/10.1371/journal.pone.0004942.
Vulpis, E., Cecere, F., Molfetta, R., Soriani, A., Fionda, C., Peruzzi, G., et al. (2017). Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: role of HSP70/TLR2/NF-kB axis. Oncoimmunology, 6, e1279372. https://doi.org/10.1080/2162402X.2017.1279372.
Budnik, V., Ruiz-Cañada, C., & Wendler, F. (2016). Extracellular vesicles round off communication in the nervous system. Nature Reviews. Neuroscience, 17, 160–172. https://doi.org/10.1038/nrn.2015.29.
Gong, J., Körner, R., Gaitanos, L., & Klein, R. (2016). Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. The Journal of Cell Biology, 214, 35–44. https://doi.org/10.1083/jcb.201601085.
Fitzgerald, T. L., & McCubrey, J. A. (2014). Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv Biol Regul, 56, 45–50. https://doi.org/10.1016/j.jbior.2014.05.001.
Cherciu, I., Bărbălan, A., Pirici, D., Mărgăritescu, C., & Săftoiu, A. (2014). Stem cells, colorectal cancer and cancer stem cell markers correlations. Current Health Sciences Journal, 40(3), 153–161. https://doi.org/10.12865/CHSJ.40.03.01.
Gesierich, S., Paret, C., Hildebrand, D., Weitz, J., Zgraggen, K., Schmitz-Winnenthal, F. H., et al. (2005). Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: impact on cell motility. Clinical Cancer Research, 11(8), 2840–2852.
Claas, C., Wahl, J., Orlicky, D. J., Karaduman, H., Schnölzer, M., Kempf, T., et al. (2005). The tetraspanin D6.1A and its molecular partners on rat carcinoma cells. The Biochemical Journal, 389(Pt 1), 99–110.
Kanatsu-Shinohara, M., Takashima, S., Ishii, K., & Shinohara, T. (2011). Dynamic changes in EPCAM expression during spermatogonial stem cell differentiation in the mouse testis. PLoS One, 6(8), e23663. https://doi.org/10.1371/journal.pone.0023663.
Le Naour, F., André, M., Greco, C., Billard, M., Sordat, B., Emile, J. F., et al. (2006). Profiling of the tetraspanin web of human colon cancer cells. Molecular & Cellular Proteomics, 5(5), 845–857.
Margadant, C., Frijns, E., Wilhelmsen, K., & Sonnenberg, A. (2008). Regulation of hemidesmosome disassembly by growth factor receptors. Current Opinion in Cell Biology, 20(5), 589–596. https://doi.org/10.1016/j.ceb.2008.05.001.
Ladwein, M., Pape, U. F., Schmidt, D. S., Schnölzer, M., Fiedler, S., Langbein, L., et al. (2005). The cell-cell adhesion molecule EpCAM interacts directly with the tight junction protein claudin-7. Experimental Cell Research, 309(2), 345–357.
Kuhn, S., Koch, M., Nübel, T., Ladwein, M., Antolovic, D., Klingbeil, P., et al. (2007). A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Molecular Cancer Research, 5(6), 553–567.
Okada, T., Nakamura, T., Watanabe, T., Onoda, N., Ashida, A., Okuyama, R., et al. (2014). Coexpression of EpCAM, CD44 variant isoforms and claudin-7 in anaplastic thyroid carcinoma. PLoS One, 9(4), e94487. https://doi.org/10.1371/journal.pone.0094487.
Wu, C. J., Mannan, P., Lu, M., & Udey, M. C. (2013). Epithelial cell adhesion molecule (EpCAM) regulates claudin dynamics and tight junctions. The Journal of Biological Chemistry, 288(17), 12253–12268. https://doi.org/10.1074/jbc.M113.457499.
Matzke-Ogi, A., Jannasch, K., Shatirishvili, M., Fuchs, B., Chiblak, S., Morton, J., et al. (2016). Inhibition of tumor growth and metastasis in pancreatic cancer models by interference with CD44v6 signaling., 150(2), Gastroenterology, 513–Gastroent525.e10. https://doi.org/10.1053/j.gastro.2015.10.020.
Parton, R. G., & del Pozo, M. A. (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nature Reviews. Molecular Cell Biology, 14(2), 98–112. https://doi.org/10.1038/nrm3512.
Lampe, M., Vassilopoulos, S., & Merrifield, C. (2016). Clathrin coated pits, plaques and adhesion. Journal of Structural Biology, 196(1), 48–56. https://doi.org/10.1016/j.jsb.2016.07.009.
Marsh, D., Horváth, L. I., Swamy, M. J., Mantripragada, S., & Kleinschmidt, J. H. (2002). Interaction of membrane-spanning proteins with peripheral and lipid-anchored membrane proteins: perspectives from protein-lipid interactions (review). Molecular Membrane Biology, 19(4), 247–255.
Bottini, M., Mebarek, S., Anderson, K. L., Strzelecka-Kiliszek, A., Bozycki, L., Simão, A. M. S., et al. (2018). Matrix vesicles from chondrocytes and osteoblasts: their biogenesis, properties, functions and biomimetic models. Biochimica et Biophysica Acta, 1862(3), 532–546. https://doi.org/10.1016/j.bbagen.2017.11.005.
Andreu, Z., & Yáñez-Mó, M. (2014). Tetraspanins in extracellular vesicle formation and function. Frontiers in Immunology, 5, 442. https://doi.org/10.3389/fimmu.2014.00442.
Stuffers, S., Sem Wegner, C., Stenmark, H., & Brech, A. (2009). Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic, 925–937. https://doi.org/10.1111/j.1600-0854.2009.00920.x.
van Niel, G., Bergam, P., Di Cicco, A., Hurbain, I., Lo Cicero, A., Dingli, F., et al. (2015). Apolipoprotein E regulates amyloid formation within endosomes of pigment cells. Cell Reports, 13(1), 43–51. https://doi.org/10.1016/j.celrep.2015.08.057.
Buschow, S. I., Nolte-'t Hoen, E. N., van Niel, G., Pols, M. S., ten Broeke, T., Lauwen, M., et al. (2009). MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic, 10(10), 1528–1542. https://doi.org/10.1111/j.1600-0854.2009.00963.x.
Zimmerman, B., Kelly, B., McMillan, B. J., Seegar, T. C. M., Dror, R. O., Kruse, A. C., et al. (2016). Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell, 167(4), 1041–1051.e11. https://doi.org/10.1016/j.cell.2016.09.056.
Odintsova, E., van Niel, G., Conjeaud, H., Raposo, G., Iwamoto, R., Mekada, E., et al. (2013). Metastasis suppressor tetraspanin CD82/KAI1 regulates ubiquitylation of epidermal growth factor receptor. The Journal of Biological Chemistry, 288(36), 26323–26334. https://doi.org/10.1074/jbc.M112.439380.
Gräßel, L., Fast, L. A., Scheffer, K. D., Boukhallouk, F., Spoden, G. A., Tenzer, S., et al. (2016). The CD63-syntenin-1 complex controls post-endocytic trafficking of oncogenic human papillomaviruses. Scientific Reports, 6, 32337. https://doi.org/10.1038/srep32337.
Li, X., Zhao, H., Gu, J., & Zheng, L. (2015). Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis. International Journal of Clinical and Experimental Pathology, 8(10), 12084–12092 eCollection 2015.
Schulze, U., Brast, S., Grabner, A., Albiker, C., Snieder, B., Holle, S., et al. (2017). Tetraspanin CD63 controls basolateral sorting of organic cation transporter 2 in renal proximal tubules. The FASEB Journal, 31(4), 1421–1433. https://doi.org/10.1096/fj.201600901R.
Zumaquero, E., Muñoz, P., Cobo, M., Lucena, G., Pavón, E. J., Martín, A., et al. (2010). Exosomes from human lymphoblastoid B cells express enzymatically active CD38 that is associated with signaling complexes containing CD81, Hsc-70 and Lyn. Experimental Cell Research, 316(16), 2692–2706. https://doi.org/10.1016/j.yexcr.2010.05.032.
Erb, U., Zhao, K., Wang, Z., Xiao, L., & Zöller, M. (2017). Murine and human pancreatic tumor exosome recovery in mouse serum: diagnostic and prognostic potential and target cell delivery. Cancer Letters, 403, 1–12. https://doi.org/10.1016/j.canlet.2017.06.005.
Hurwitz, S. N., Nkosi, D., Conlon, M. M., York, S. B., Liu, X., Tremblay, D. C., et al. (2017). CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling. Journal of Virology, 91(5), e02251–e02216. https://doi.org/10.1128/JVI.02251-16.
Kristiansen, G., Sammar, M., & Altevogt, P. (2004). Tumour biological aspects of CD24, a mucin-like adhesion molecule. Journal of Molecular Histology, 35(3), 255–262.
Corbeil, D., Joester, A., Fargeas, C. A., Jászai, J., Garwood, J., Hellwig, A., et al. (2009). Expression of distinct splice variants of the stem cell marker prominin-1 (CD133) in glial cells. Glia, 57(8), 860–874. https://doi.org/10.1002/glia.20812.
Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., et al. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science, 319(5867), 1244–1247. https://doi.org/10.1126/science.1153124.
Badawy, S. M. M., Okada, T., Kajimoto, T., Hirase, M., Matovelo, S. A., Nakamura, S., et al. (2018). Extracellular α-synuclein drives sphingosine 1-phosphate receptor subtype 1 out of lipid rafts, leading to impaired inhibitory G-protein signaling. The Journal of Biological Chemistry, 293(21), 8208–8216. https://doi.org/10.1074/jbc.RA118.001986.
Hartman, N. C., & Groves, J. T. (2011). Signaling clusters in the cell membrane. Current Opinion in Cell Biology, 23(4), 370–376. https://doi.org/10.1016/j.ceb.2011.05.003.
Gonnord, P., Blouin, C. M., & Lamaze, C. (2012). Membrane trafficking and signaling: two sides of the same coin. Seminars in Cell & Developmental Biology, 23(2), 154–164. https://doi.org/10.1016/j.semcdb.2011.11.002.
Head, B. P., Patel, H. H., & Insel, P. A. (2014). Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochimica et Biophysica Acta, 1838(2), 532–545. https://doi.org/10.1016/j.bbamem.2013.07.018.
Melo, S. A., Sugimoto, H., O'Connell, J. T., Kato, N., Villanueva, A., Vidal, A., et al. (2014). Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell, 26(5), 707–721. https://doi.org/10.1016/j.ccell.2014.09.005.
Nag, A., & Steitz, J. A. (2012). Tri-snRNP-associated proteins interact with subunits of the TRAMP and nuclear exosome complexes, linking RNA decay and pre-mRNA splicing. RNA Biology, 9(3), 334–342. https://doi.org/10.4161/rna.19431.
Chang, A. Y., Castel, S. E., Ernst, E., Kim, H. S., & Martienssen, R. A. (2017). The conserved RNA binding cyclophilin, Rct1, regulates small RNA biogenesis and splicing independent of heterochromatin assembly. Cell Reports, 19(12), 2477–2489. https://doi.org/10.1016/j.celrep.2017.05.086.
Tran, N. (2016). Cancer exosomes as miRNA factories. Trends Cancer, 2(7), 329–331. https://doi.org/10.1016/j.trecan.2016.05.008.
Gao, T., Shu, J., & Cui, J. (2018). A systematic approach to RNA-associated motif discovery. BMC Genomics, 19(1), 146. https://doi.org/10.1186/s12864-018-4528-x.
Shapiro, I. M., Landis, W. J., & Risbud, M. V. (2015). Matrix vesicles: are they anchored exosomes? Bone, 79, 29–36. https://doi.org/10.1016/j.bone.2015.05.013.
Belov, L., Matic, K. J., Hallal, S., Best, O. G., Mulligan, S. P., & Christopherson, R. I. (2016). Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J Extracell Vesicles, 5, 25355. https://doi.org/10.3402/jev.v5.25355.
Chase, S. D., Magnani, J. L., & Simon, S. I. (2012). E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Annals of Biomedical Engineering, 40(4), 849–859. https://doi.org/10.1007/s10439-011-0507-y.
Levy, S., Todd, S. C., & Maecker, H. T. (1998). CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annual Review of Immunology, 16, 89–109.
Zech, D., Rana, S., Büchler, M. W., & Zöller, M. (2012). Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Communication and Signaling: CCS, 10(1), 37. https://doi.org/10.1186/1478-811X-10-37.
Lau, L. M., Wee, J. L., Wright, M. D., Moseley, G. W., Hogarth, P. M., Ashman, L. K., et al. (2004). The tetraspanin superfamily member CD151 regulates outside-in integrin alphaIIbbeta3 signaling and platelet function. Blood, 104(8), 2368–2375.
Zhao, K., Erb, U., Hackert, T., Zöller, M., & Yue, S. (2018). Distorted leukocyte migration, angiogenesis, wound repair and metastasis in Tspan8 and Tspan8/CD151 double knockout mice indicate complementary activities of Tspan8 and CD51. Biochimica et Biophysica Acta, 1865(2), 379–391. https://doi.org/10.1016/j.bbamcr.2017.11.007.
Yue, S., Mu, W., Erb, U., & Zöller, M. (2015). The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget, 6(4), 2366–2384.
Kyuno, D., Zhao, K., Bauer, N., Ryschich, E., & Zöller, M. (2018). Therapeutic targeting cancer-initiating cell markers by exosome miRNA: efficacy and functional consequences exemplified for claudin7 and EpCAM. Translational Oncology, 12(2), 191–199. https://doi.org/10.1016/j.tranon.2018.08.021.
Henne, W. M., Liou, J., & Emr, S. D. (2015). Molecular mechanisms of inter-organelle ER-PM contact sites. Current Opinion in Cell Biology, 35, 123–130. https://doi.org/10.1016/j.ceb.2015.05.001.
Blank, F., Wehrli, M., Lehmann, A., Baum, O., Gehr, P., von Garnier, C., et al. (2011). Macrophages and dendritic cells express tight junction proteins and exchange particles in an in vitro model of the human airway wall. Immunobiology, 216(1–2), 86–95. https://doi.org/10.1016/j.imbio.2010.02.006.
Nelson, G. M., Padera, T. P., Garkavtsev, I., Shioda, T., & Jain, R. K. (2007). Differential gene expression of primary cultured lymphatic and blood vascular endothelial cells. Neoplasia, 9(12), 1038–1045.
Nogués, L., Benito-Martin, A., Hergueta-Redondo, M., & Peinado, H. (2018). The influence of tumour-derived extracellular vesicles on local and distal metastatic dissemination. Molecular Aspects of Medicine, 60, 15–26. https://doi.org/10.1016/j.mam.2017.11.012.
Li, M., Lu, Y., Xu, Y., Wang, J., Zhang, C., Du, Y., et al. (2018). Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene, S0378-1119(18), 30787-X. https://doi.org/10.1016/j.gene.2018.07.018.
Shen, L., Weber, C. R., & Turner, J. R. (2008). The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. The Journal of Cell Biology, 181(4), 683–695. https://doi.org/10.1083/jcb.200711165.
Rilla, K., Siiskonen, H., Tammi, M., & Tammi, R. (2014). Hyaluronan-coated extracellular vesicles—a novel link between hyaluronan and cancer. Advances in Cancer Research, 123, 121–148. https://doi.org/10.1016/B978-0-12-800092-2.00005-8.
Purushothaman, A., Bandari, S. K., Liu, J., Mobley, J. A., Brown, E. E., & Sanderson, R. D. (2016). Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. The Journal of Biological Chemistry, 291(4), 1652–1663. https://doi.org/10.1074/jbc.M115.686295.
Dismuke, W. M., Klingeborn, M., & Stamer, W. D. (2016). Mechanism of fibronectin binding to human trabecular meshwork exosomes and its modulation by dexamethasone. PLoS One, 11(10), e0165326. https://doi.org/10.1371/journal.pone.0165326 eCollection 2016.
Shimoda, M., & Khokha, R. (2013). Proteolytic factors in exosomes. Proteomics, 13(10–11), 1624–1636. https://doi.org/10.1002/pmic.201200458.
Sevenich, L., & Joyce, J. A. (2014). Pericellular proteolysis in cancer. Genes & Development, 28(21), 2331–2347. https://doi.org/10.1101/gad.250647.114.
Silva, A. M., Teixeira, J. H., Almeida, M. I., Gonçalves, R. M., Barbosa, M. A., & Santos, S. G. (2017). Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration. European Journal of Pharmaceutical Sciences, 98, 86–95. https://doi.org/10.1016/j.ejps.2016.09.017.
Than, U. T. T., Guanzon, D., Leavesley, D., & Parker, T. (2017). association of extracellular membrane vesicles with cutaneous wound healing. International Journal of Molecular Sciences, 18(5), E956. https://doi.org/10.3390/ijms18050956.
Bandari, S. K., Purushothaman, A., Ramani, V. C., Brinkley, G. J., Chandrashekar, D. S., Varambally, S., et al. (2018). Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biology, 65, 104–118. https://doi.org/10.1016/j.matbio.2017.09.001.
Shi, F., & Sottile, J. (2011). MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. Journal of Cell Science, 124(Pt 23), 4039–4050. https://doi.org/10.1242/jcs.087858.
Jessen, T. N., & Jessen, J. R. (2017). VANGL2 interacts with integrin αv to regulate matrix metalloproteinase activity and cell adhesion to the extracellular matrix. Experimental Cell Research, 361(2), 265–276. https://doi.org/10.1016/j.yexcr.2017.10.026.
Nalivaeva, N. N., Belyaev, N. D., Kerridge, C., & Turner, A. J. (2014). Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer’s disease. Frontiers in Aging Neuroscience, 6, 235. https://doi.org/10.3389/fnagi.2014.00235.
Jung, T., Gross, W., & Zöller, M. (2011). CD44v6 coordinates tumor matrix-triggered motility and apoptosis resistance. The Journal of Biological Chemistry, 286(18), 15862–15874. https://doi.org/10.1074/jbc.M110.208421.
Quesenberry, P. J., Aliotta, J., Deregibus, M. C., & Camussi, G. (2015). Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Research & Therapy, 6, 153. https://doi.org/10.1186/s13287-015-0150-x.
Kanada, M., Bachmann, M. H., Hardy, J. W., Frimannson, D. O., Bronsart, L., Wang, A., et al. (2015). Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proceedings of the National Academy of Sciences of the United States of America, 112(12), E1433–E1442. https://doi.org/10.1073/pnas.1418401112.
Shin, S. J., Smith, J. A., Rezniczek, G. A., Pan, S., Chen, R., Brentnall, T. A., et al. (2013). Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America, 110(48), 19414–19419. https://doi.org/10.1073/pnas.1309720110.
Rana, S., Malinowska, K., & Zöller, M. (2013). Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia, 15(3), 281–295.
Wang, Z., Zhao, K., Hackert, T., & Zöller, M. (2018). CD44/CD44v6 a reliable companion in cancer-initiating cell maintenance and tumor progression. Frontiers in Cell and Development Biology, 6, 97. https://doi.org/10.3389/fcell.2018.00097.
Song, X., Ding, Y., Liu, G., Yang, X., Zhao, R., Zhang, Y., et al. (2016). Cancer cell-derived exosomes induce mitogen-activated protein kinase-dependent monocyte survival by transport of functional receptor tyrosine kinases. The Journal of Biological Chemistry, 291(16), 8453–8464. https://doi.org/10.1074/jbc.M116.716316.
Zhang, H., Deng, T., Liu, R., Bai, M., Zhou, L., Wang, X., et al. (2017). Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nature Communications, 8, 15016. https://doi.org/10.1038/ncomms15016.
Bendinelli, P., Maroni, P., Matteucci, E., & Desiderio, M. A. (2017). Epigenetic regulation of HGF/Met receptor axis is critical for the outgrowth of bone metastasis from breast carcinoma. Cell Death & Disease, 8(2), e2578. https://doi.org/10.1038/cddis.2016.403.
Yang, W. W., Yang, L. Q., Zhao, F., Chen, C. W., Xu, L. H., Fu, J., et al. (2017). Epiregulin promotes lung metastasis of salivary adenoid cystic carcinoma. Theranostics, 7(15), 3700–3714. https://doi.org/10.7150/thno.19712.
Kwon, S. H., Liu, K. D., & Mostov, K. E. (2014). Intercellular transfer of GPRC5B via exosomes drives HGF-mediated outward growth. Current Biology, 24(2), 199–204. https://doi.org/10.1016/j.cub.2013.12.010.
Fuchs, K., Hippe, A., Schmaus, A., Homey, B., Sleeman, J. P., & Orian-Rousseau, V. (2013). Opposing effects of high- and low-molecular weight hyaluronan on CXCL12-induced CXCR4 signaling depend on CD44. Cell Death & Disease, 4, e819. https://doi.org/10.1038/cddis.2013.364.
Roscic-Mrkic, B., Fischer, M., Leemann, C., Manrique, A., Gordon, C. J., Moore, J. P., et al. (2003). RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood, 102(4), 1169–1177.
Zhu, B., Suzuki, K., Goldberg, H. A., Rittling, S. R., Denhardt, D. T., McCulloch, C. A., et al. (2004). Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. Journal of Cellular Physiology, 198(1), 155–167.
Che, S. P. Y., Park, J. Y., & Stokol, T. (2017). Tissue factor-expressing tumor-derived extracellular vesicles activate quiescent endothelial cells via protease-activated receptor-1. Frontiers in Oncology, 7, 261. https://doi.org/10.3389/fonc.2017.00261.
Gilliam, D. T., Menon, V., Bretz, N. P., & Pruszak, J. (2017). The CD24 surface antigen in neural development and disease. Neurobiology of Disease, 99, 133–144. https://doi.org/10.1016/j.nbd.2016.12.011.
Lim, J., Lee, K. M., Shim, J., & Shin, I. (2014). CD24 regulates stemness and the epithelial to mesenchymal transition through modulation of Notch1 mRNA stability by p38MAPK. Archives of Biochemistry and Biophysics, 558, 120–126. https://doi.org/10.1016/j.abb.2014.06.022.
Lee, T. K., Castilho, A., Cheung, V. C., Tang, K. H., Ma, S., & Ng, I. O. (2011). CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell, 9(1), 50–63. https://doi.org/10.1016/j.stem.2011.06.005.
Takenobu, H., Shimozato, O., Nakamura, T., Ochiai, H., Yamaguchi, Y., Ohira, M., et al. (2011). CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene, 30(1), 97–105. https://doi.org/10.1038/onc.2010.383.
Zhang, M., Liu, Y., Feng, H., Bian, X., Zhao, W., Yang, Z., et al. (2013). CD133 affects the invasive ability of HCT116 cells by regulating TIMP-2. The American Journal of Pathology, 182(2), 565–576. https://doi.org/10.1016/j.ajpath.2012.10.015.
Lee, J. W., Lee, Y. C., Na, S. Y., Jung, D. J., & Lee, S. K. (2001). Transcriptional coregulators of the nuclear receptor superfamily: coactivators and corepressors. Cellular and Molecular Life Sciences, 58(2), 289–297.
Mazzi, S., Lordier, L., Debili, N., Raslova, H., & Vainchenker, W. (2018). Megakaryocyte and polyploidization. Experimental Hematology, 57, 1–13. https://doi.org/10.1016/j.exphem.2017.10.001.
Heneberg, P. (2016). Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Critical Reviews in Oncology/Hematology, 97, 303–311. https://doi.org/10.1016/j.critrevonc.2015.09.008.
Cho, J. A., Park, H., Lim, E. H., & Lee, K. W. (2012). Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. International Journal of Oncology, 40(1), 130–138. https://doi.org/10.3892/ijo.2011.1193.
Record, M., Poirot, M., & Silvente-Poirot, S. (2014). Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie, 96, 67–74. https://doi.org/10.1016/j.biochi.2013.06.016.
Fonseca, P., Vardaki, I., Occhionero, A., & Panaretakis, T. (2016). Metabolic and signaling functions of cancer cell-derived extracellular vesicles. International Review of Cell and Molecular Biology, 326, 175–199. https://doi.org/10.1016/bs.ircmb.2016.04.004.
Skotland, T., Sandvig, K., & Llorente, A. (2017). Lipids in exosomes: current knowledge and the way forward. Progress in Lipid Research, 66, 30–41. https://doi.org/10.1016/j.plipres.2017.03.001.
García-González, V., Díaz-Villanueva, J. F., Galindo-Hernández, O., Martínez-Navarro, I., Hurtado-Ureta, G., & Pérez-Arias, A. A. (2018). Ceramide metabolism balance, a multifaceted factor in critical steps of breast cancer development. International Journal of Molecular Sciences, 19(9), E2527. https://doi.org/10.3390/ijms19092527.
Hsu, M. C., & Hung, W. C. (2018). Pyruvate kinase M2 fuels multiple aspects of cancer cells: from cellular metabolism, transcriptional regulation to extracellular signaling. Molecular Cancer, 17(1), 35. https://doi.org/10.1186/s12943-018-0791-3.
Alcayaga-Miranda, F., González, P. L., Lopez-Verrilli, A., Varas-Godoy, M., Aguila-Díaz, C., Contreras, L., et al. (2016). Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget, 7(28), 44462–44477. https://doi.org/10.18632/oncotarget.9852.
van Balkom, B. W., Eisele, A. S., Pegtel, D. M., Bervoets, S., & Verhaar, M. C. (2015). Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles, 4, 26760. https://doi.org/10.3402/jev.v4.26760.
Deng, X., Wu, B., Xiao, K., Kang, J., Xie, J., Zhang, X., et al. (2015). MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cellular Physiology and Biochemistry, 35(1), 71–82. https://doi.org/10.1159/000369676.
Rutnam, Z. J., & Yang, B. B. (2012). The non-coding 3’ UTR of CD44 induces metastasis by regulating extracellular matrix functions. Journal of Cell Science, 125(Pt 8), 2075–2085. https://doi.org/10.1242/jcs100818.
Li, J., & Lam, M. (2015). Reproducibility project: cancer biology. Registered report: the microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Elife, 4, e06434. https://doi.org/10.7554/eLife.06434.
Li, X., He, J., Shao, M., Cui, B., Peng, F., Li, J., et al. (2018). Downregulation of miR-218-5p promotes invasion of oral squamous cell carcinoma cells via activation of CD44-ROCK signaling. Biomedicine & Pharmacotherapy, 106, 646–654. https://doi.org/10.1016/j.biopha.2018.06.151.
Al-Othman, N., Hammad, H., & Ahram, M. (2018). Dihydrotestosterone regulates expression of CD44 via miR-328-3p in triple-negative breast cancer cells. Gene, S0378-1119(18), 30755–30758. https://doi.org/10.1016/j.gene.2018.06.094.
Wu, Z. S., Wu, Q., Wang, C. Q., Wang, X. N., Huang, J., Zhao, J. J., et al. (2011). miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer, 117, 2842–2852. https://doi.org/10.1002/cncr.25860.
Takahashi, H., Ohkuchi, A., Kuwata, T., Usui, R., Baba, Y., Suzuki, H., et al. (2017). Endogenous and exogenous miR-520c-3p modulates CD44-mediated extravillous trophoblast invasion. Placenta, 50, 25–31. https://doi.org/10.1016/j.placenta.2016.12.016.
Shen, W. W., Zeng, Z., Zhu, W. X., & Fu, G. H. (2013). MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl), 91(8), 989–1000. https://doi.org/10.1007/s00109-013-1037-x.
Tsuji, S., Kawasaki, Y., Furukawa, S., Taniue, K., Hayashi, T., Okuno, M., et al. (2014). The miR-363-GATA6-Lgr5 pathway is critical for colorectal tumourigenesis. Nature Communications, 5, 3150. https://doi.org/10.1038/ncomms4150.
Zhou, M. K., Liu, X. J., Zhao, Z. G., & Cheng, Y. M. (2015). MicroRNA-100 functions as a tumor suppressor by inhibiting Lgr5 expression in colon cancer cells. Molecular Medicine Reports, 11(4), 2947–2952. https://doi.org/10.3892/mmr.2014.3052.
Ostenfeld, M. S., Jensen, S. G., Jeppesen, D. K., Christensen, L. L., Thorsen, S. B., Stenvang, J., et al. (2016). miRNA profiling of circulating EpCAM(+) extracellular vesicles: promising biomarkers of colorectal cancer. J Extracell Vesicles, 5, 31488. https://doi.org/10.3402/jev.v5.31488.
Ji, H., Chen, M., Greening, D. W., He, W., Rai, A., Zhang, W., et al. (2014). Deep sequencing of RNA from three different extracellular vesicle (EV) subtypes released from the human LIM1863 colon cancer cell line uncovers distinct miRNA-enrichment signatures. PLoS One, 9(10), e110314. https://doi.org/10.1371/journal.pone.0110314.
Hu, Y., Wang, J., Qian, J., Kong, X., Tang, J., Wang, Y., et al. (2014). Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Research, 74(23), 6890–6902. https://doi.org/10.1158/0008-5472.CAN-14-0686.
Wu, X., He, X., Li, S., Xu, X., Chen, X., & Zhu, H. (2016). Long non-coding RNA ucoo2kmd.1 regulates CD44-dependent cell growth by competing for miR-211-3p in colorectal cancer. PLoS One, 11(3), e0151287. https://doi.org/10.1371/journal.pone.0151287.
Wang, R., Dong, H. X., Zeng, J., Pan, J., & Jin, X. Y. (2018). LncRNA DGCR5 contributes to CSC-like properties via modulating miR-330-5p/CD44 in NSCLC. Journal of Cellular Physiology, 233(9), 7447–7456. https://doi.org/10.1002/jcp.26590.
Ji, J., Tang, J., Deng, L., Xie, Y., Jiang, R., Li, G., et al. (2015). LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget, 6(40), 42813–42824. https://doi.org/10.18632/oncotarget.5970.
Zhang, Z.Y., Lu, Y.X., Zhang, Z.Y., Chang, Y.Y., Zheng, L., Yuan, L., et al. (2016). Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget, 7(16), 22639–22649. doi: 10.18632/oncotarget.8141.
Liu, J., Yang, C., Gu, Y., Li, C., Zhang, H., Zhang, W., et al. (2018). Knockdown of the lncRNA SNHG8 inhibits cell growth in Epstein-Barr virus-associated gastric carcinoma. Cellular & Molecular Biology Letters, 23, 17. https://doi.org/10.1186/s11658-018-0070-8.
Ren, H., Yang, X., Yang, Y., Zhang, X., Zhao, R., Wie, R., et al. (2017). Upregulation of LncRNA BCYRN1 promotes tumor progression and enhances EpCAM expression in gastric carcinoma. Oncotarget, 9(4), 4851–4861. https://doi.org/10.18632/oncotarget.23585.
Jing, N., Huang, T., Guo, H., Yang, J., Li, M., Chen, Z., et al. (2018). LncRNA CASC15 promotes colon cancer cell proliferation and metastasis by regulating the miR-4310/LGR5/Wnt/β-catenin signaling pathway. Molecular Medicine Reports, 18(2), 2269–2276. https://doi.org/10.3892/mmr.2018.9191.
Song, Y. X., Sun, J. X., Zhao, J. H., Yang, Y. C., Shi, J. X., Wu, Z. H., et al. (2017). Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nature Communications, 8(1), 289. https://doi.org/10.1038/s41467-017-00304-1.
Chen, T., Xue, H., Lin, R., & Huang, Z. (2017). MiR-34c and PlncRNA1 mediated the function of intestinal epithelial barrier by regulating tight junction proteins in inflammatory bowel disease. Biochemical and Biophysical Research Communications, 486(1), 6–13. https://doi.org/10.1016/j.bbrc.2017.01.115.
Chen, M., Xu, R., Ji, H., Greening, D. W., Rai, A., Izumikawa, K., et al. (2016). Transcriptome and long noncoding RNA sequencing of three extracellular vesicle subtypes released from the human colon cancer LIM1863 cell line. Scientific Reports, 6, 38397. https://doi.org/10.1038/srep38397.
Wang, F., Tian, X., Zhou, J., Wang, G., Yu, W., Li, Z., et al. (2018). A three-lncRNA signature for prognosis prediction of acute myeloid leukemia in patients. Molecular Medicine Reports, 18(2), 1473–1484. https://doi.org/10.3892/mmr.2018.9139.
Xie, S., Ge, Q., Wang, X., Sun, X., & Kang, Y. (2018). Long non-coding RNA ZFAS1 sponges miR-484 to promote cell proliferation and invasion in colorectal cancer. Cell Cycle, 17(2), 154–161. https://doi.org/10.1080/15384101.2017.1407895.
Li, N., Sun, Z. H., Fang, M., Xin, J. Y., & Wan, C. Y. (2017). Long non-coding RNA ZFAS1 sponges miR-486 to promote osteosarcoma cells progression and metastasis in vitro and vivo. Oncotarget, 8(61), 104160–104170. https://doi.org/10.18632/oncotarget.22032.
Xu, W., He, L., Li, Y., Tan, Y., Zhang, F., & Xu, H. (2018). Silencing of lncRNA ZFAS1 inhibits malignancies by blocking Wnt/β-catenin signaling in gastric cancer cells. Bioscience, Biotechnology, and Biochemistry, 82(3), 456–465. https://doi.org/10.1080/09168451.2018.1431518.
Yang, C. H., Zhang, X. Y., Zhou, L. N., Wan, Y., Song, L. L., Gu, W. L., et al. (2018). LncRNA SNHG8 participates in the development of endometrial carcinoma through regulating c-MET expression by miR-152. European Review for Medical and Pharmacological Sciences, 22(6), 1629–1637. https://doi.org/10.26355/eurrev_201803_14698.
Chen, Z., Bu, N., Qiao, X., Zuo, Z., Shu, Y., Liu, Z., et al. (2018). Forkhead box M1 transcriptionally regulates the expression of long noncoding RNAs Snhg8 and Gm26917 to promote proliferation and survival of muscle satellite cells. Stem Cells. https://doi.org/10.1002/stem.2824.
Wan, L., Kong, J., Tang, J., Wu, Y., Xu, E., Lai, M., et al. (2016). HOTAIRM1 as a potential biomarker for diagnosis of colorectal cancer functions the role in the tumour suppressor. Journal of Cellular and Molecular Medicine, 20(11), 2036–2044. https://doi.org/10.1111/jcmm.12892.
Chen, Z. H., Wang, W. T., Huang, W., Fang, K., Sun, Y. M., Liu, S. R., et al. (2017). The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death and Differentiation, 24(2), 212–224. https://doi.org/10.1038/cdd.2016.111.
Marín-Béjar, O., Mas, A. M., González, J., Martinez, D., Athie, A., Morales, X., et al. (2017). The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biology, 18(1), 202. https://doi.org/10.1186/s13059-017-1331-y.
Li, Y., Huang, S., Li, Y., Zhang, W., He, K., Zhao, M., et al. (2016). Decreased expression of LncRNA SLC25A25-AS1 promotes proliferation, chemoresistance, and EMT in colorectal cancer cells. Tumour Biology, 37(10), 14205–14215. https://doi.org/10.1007/s13277-016-5254-0.
Jiao, F., Hu, H., Han, T., Yuan, C., Wang, L., Jin, Z., et al. (2015). Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. International Journal of Molecular Sciences, 16(4), 6677–6693. https://doi.org/10.3390/ijms16046677.
Geng, H., Bu, H. F., Liu, F., Wu, L., Pfeifer, K., Chou, P. M., et al. (2018). In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of h19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology, 155(1), 144–155. https://doi.org/10.1053/j.gastro.2018.03.058.
Abbreviations
A-ISC active ISC, AML acute myeloid leukemia, ASC adult stem cells, BMC bone marrow cells, CAF cancer-associated fibroblasts, ceRNA competing endogenous RNA, CIC cancer-initiating cells/cancer stem cells, CoCa colorectal cancer, DC dendritic cells, DS deep sequencing, EBV Epstein–Barr virus, EC endothelial cells, ECM extracellular matrix, EE early endosome, EMT epithelial–mesenchymal transition, ERM ezrin, radixin, moesin, ESC embryonic stem cells, ESCRT endosomal sorting complex required for transport, EV extracellular vesicles, Exo exosome, GAG glycosaminoglycan, GEM glycolipid-enriched membrane domains, GPCR G protein-coupled receptor, HCC hepatocellular carcinoma, hiPSC human-induced pluripotent SC, ICD intracellular domain, ILV intraluminal vesicle, ISC intestinal SC, kd knockdown, ko knockout, LDL low-density lipoprotein, LN laminin, lnc long nc, LNC lymph node cells, Mϕ macrophage, MHC major histocompatibility complex, miRNA microRNA, MS mass spectrometry, MVB multivesicular body, nc noncoding, NK natural killer cells, NSCLC nonsmall cell lung carcinoma, PaCa pancreatic cancer, PSC pluripotent SC, R receptor, RA retinoic acid, RISC RNA-induced silencing complex, ROS reactive oxygen species, RTK receptor tyrosine kinase, Q-ISC quiescent ISC, SC stem cells, TEM tetraspanin- and glycolipid-enriched membrane microdomain, TEX tumor exosomes, TF transcription factor, TJ tight junction
Funding
This work was supported by the National Natural Science Foundation of China (ZW, NSFC. 81702877) and the German Cancer Research Aid (MZ, 110836). The funding had no impact on the design of the study and on collection, analysis and interpretation of data, and on writing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Microarray
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE119031, GSE119032, -GSE 11903, -GSE50632, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE120185).
Deep sequencing
ENA database accession No: PRJEB25446
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 97 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Zöller, M. Exosomes, metastases, and the miracle of cancer stem cell markers. Cancer Metastasis Rev 38, 259–295 (2019). https://doi.org/10.1007/s10555-019-09793-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-019-09793-6