Abstract
Low socioeconomic status (SES) is associated with early onset of chronic diseases and reduced life expectancy. The involvement of neighborhood-level factors in defining cancer risk and outcomes for marginalized communities has been an active area of research for decades. Yet, the biological processes that underlie the impact of SES on chronic health conditions, such as cancer, remain poorly understood. To date, limited studies have shown that chronic life stress is more prevalent in low SES communities and can affect important molecular processes implicated in tumor biology such as DNA methylation, inflammation, and immune response. Further efforts to elucidate how neighborhood-level factors function physiologically to worsen cancer outcomes for disadvantaged communities are underway. This review provides an overview of the current literature on how socioenvironmental factors within neighborhoods contribute to more aggressive tumor biology, specifically in Black U.S. women and men, including the impact of environmental pollutants, neighborhood deprivation, social isolation, structural racism, and discrimination. We also summarize commonly used methods to measure deprivation, discrimination, and structural racism at the neighborhood-level in cancer health disparities research. Finally, we offer recommendations to adopt a multi-faceted intersectional approach to reduce cancer health disparities and develop effective interventions to promote health equity.

Similar content being viewed by others
Data availability
All studies cited in this review are publicly available.
References
Lawrence WR, McGee-Avila JK, Vo JB, Luo Q, Chen Y, Inoue-Choi M, de Berrington Gonzalez A, Freedman ND, Shiels MS (2022) Trends in cancer mortality among black individuals in the US from 1999 to 2019. JAMA Oncol 8(8):1184–1189
Williams DR, Collins C (2001) Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 116(5):404–416
Annesi CA, Poulson MR, Mak KS, Tapan U, Dechert TA, Litle VR, Suzuki K (2022) The impact of residential racial segregation on non-small cell lung cancer treatment and outcomes. Ann Thorac Surg 113(4):1291–1298
Zhou Y, Bemanian A, Beyer KM (2017) Housing discrimination, residential racial segregation, and colorectal cancer survival in southeastern wisconsin. Cancer Epidemiol Biomarkers Prev 26(4):561–568
Ojinnaka CO, Luo W, Ory MG, McMaughan D, Bolin JN (2017) Disparities in surgical treatment of early-stage breast cancer among female residents of Texas: the role of racial residential segregation. Clin Breast Cancer 17(2):e43–e52
Carmona M (2019) Place value: place quality and its impact on health, social, economic and environmental outcomes. J Urban Des 24(1):1–48
Robinette JW, Charles ST, Gruenewald TL (2017) Neighborhood socioeconomic status and health: a longitudinal analysis. J Community Health 42(5):865–871
Diez Roux AV, Mair C (2010) Neighborhoods and health. Ann N Y Acad Sci 1186:125–145
Kc M, Oral E, Rung AL, Trapido EJ, Rozek LS, Fontham ETH, Bensen JT, Farnan L, Steck SE, Song L et al (2022) Neighborhood deprivation and risk of mortality among men with prostate cancer: findings from a long-term follow-up study. Prostate 82(7):783–792
Smith BP, Madak-Erdogan Z (2018) Urban neighborhood and residential factors associated with breast cancer in African American Women: a systematic review. Horm Cancer 9(2):71–81
Shen J, Fuemmeler BF, Sheppard VB, Bear HD, Song R, Chow WH, Zhao H (2022) Neighborhood disadvantage and biological aging biomarkers among breast cancer patients. Sci Rep 12(1):11006
Babatunde OA, Zahnd WE, Eberth JM, Lawson AB, Adams SA, Boakye EA, Jefferson MS, Allen CG, Pearce JL, Li H et al (2021) Association between neighborhood social deprivation and stage at diagnosis among breast cancer patients in South Carolina. Int J Environ Res Public Health 18(22):11824
Qin B, Babel RA, Plascak JJ, Lin Y, Stroup AM, Goldman N, Ambrosone CB, Demissie K, Hong CC, Bandera EV et al (2021) Neighborhood social environmental factors and breast cancer subtypes among Black women. Cancer Epidemiol Biomark Prev 30(2):344–350
Krieger N, Wright E, Chen JT, Waterman PD, Huntley ER, Arcaya M (2020) Cancer stage at diagnosis, historical redlining, and current neighborhood characteristics: breast, cervical, lung, and colorectal cancers, Massachusetts, 2001–2015. Am J Epidemiol 189(10):1065–1075
Collin LJ, Gaglioti AH, Beyer KM, Zhou Y, Moore MA, Nash R, Switchenko JM, Miller-Kleinhenz JM, Ward KC, McCullough LE (2021) Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol Biomark Prev 30(1):53–60
Plascak JJ, Beyer K, Xu X, Stroup AM, Jacob G, Llanos AAM (2022) Association between residence in historically redlined districts indicative of structural racism and racial and ethnic disparities in breast cancer outcomes. JAMA Netw Open 5(7):e2220908
Beyer KMM, Zhou Y, Laud PW, McGinley EL, Yen TWF, Jankowski C, Rademacher N, Namin S, Kwarteng J, Beltran Ponce S et al (2021) Mortgage lending bias and breast cancer survival among older women in the United States. J Clin Oncol 39(25):2749–2757
Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT (2017) Structural racism and health inequities in the USA: evidence and interventions. Lancet 389(10077):1453–1463
Abraham IE, Rauscher GH, Patel AA, Pearse WB, Rajakumar P, Burkart M, Aleem A, Dave A, Bharadwaj S, Paydary K et al (2022) Structural racism is a mediator of disparities in acute myeloid leukemia outcomes. Blood 139(14):2212–2226
Eldridge L, Berrigan D (2022) Structural racism and triple-negative breast cancer among black and white women in the United States. Health Equity 6(1):116–123
Lett E, Adekunle D, McMurray P, Asabor EN, Irie W, Simon MA, Hardeman R, McLemore MR (2022) Health equity tourism: ravaging the justice landscape. J Med Syst 46(3):17
McFarling UL (2022) ‘Health equity tourists’: How white scholars are colonizing research on health disparities. 2021, 2022(August 30, 2022)
Nelson B (2020) How structural racism can kill cancer patients: Black patients with breast cancer and other malignancies face historical inequities that are ingrained but not inevitable. In this article, the second of a 2-part series, we explore the consequences of and potential solutions to racism and inequality in cancer care. Cancer Cytopathol 128(2):83–84
Goel N, Westrick AC, Bailey ZD, Hernandez A, Balise RR, Goldfinger E, Antoni MH, Stoler J, Kesmodel SB, Kobetz EN (2022) Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg 275(4):776–783
Cheng E, Soulos PR, Irwin ML, Cespedes Feliciano EM, Presley CJ, Fuchs CS, Meyerhardt JA, Gross CP (2021) Neighborhood and individual socioeconomic disadvantage and survival among patients with nonmetastatic common cancers. JAMA Netw Open 4(12):e2139593
Unger JM, Moseley AB, Cheung CK, Osarogiagbon RU, Symington B, Ramsey SD, Hershman DL (2021) Persistent disparity: socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J Clin Oncol 39(12):1339–1348
Saini G, Ogden A, McCullough LE, Torres M, Rida P, Aneja R (2019) Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control 30(7):677–686
Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, Elo I, Burke JG, O’Campo P (2006) The development of a standardized neighborhood deprivation index. J Urban Health 83(6):1041–1062
Census Tracts and Block Numbering Areas. https://www2.census.gov/geo/pdfs/reference/GARM/Ch10GARM.pdf
Kind AJH, Buckingham WR (2018) Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med 378(26):2456–2458
Research CfHD (2022) About the neighborhood atlas. 2022 (August 30, 2022)
Glassman B (2022) Multidimensional deprivation in the United States: 2017. 2019, 2022 (August 30, 2022)
Andrews MR, Tamura K, Claudel SE, Xu S, Ceasar JN, Collins BS, Langerman S, Mitchell VM, Baumer Y, Powell-Wiley TM (2020) Geospatial analysis of Neighborhood Deprivation Index (NDI) for the United States by County. J Maps 16(1):101–112
Slotman BA, Stinchcomb DG, Powell-Wiley TM, Ostendorf DM, Saelens BE, Gorin AA, Zenk SN, Berrigan D (2022) Environmental data and methods from the Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) core measures environmental working group. Data Brief 41:108002
National Cancer Institute Surveillance EaERP (2022) Census Tract-level SES and Rurality Database (2006–2018). 2022 (August 30, 2022)
Yost K, Perkins C, Cohen R, Morris C, Wright W (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12(8):703–711
Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R (2002) Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol 156(5):471–482
Trinidad S, Brokamp C, Mor Huertas A, Beck AF, Riley CL, Rasnik E, Falcone R, Kotagal M (2022) Use of area-based socioeconomic deprivation indices: a scoping review and qualitative analysis. Health Aff 41(12):1804–1811
Krieger N, Williams DR, Moss NE (1997) Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health 18:341–378
Hardeman RR, Homan PA, Chantarat T, Davis BA, Brown TH (2022) Improving the measurement of structural racism to achieve antiracist health policy. Health Aff 41(2):179–186
Williams DR, Yan Y, Jackson JS, Anderson NB (1997) Racial Differences in physical and mental health: socio-economic status stress and discrimination. J Health Psychol 2(3):335–351
Krieger N, Sidney S (1996) Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. Am J Public Health 86(10):1370–1378
Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM (2005) Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 61(7):1576–1596
Torres-Harding SR, Andrade AL, Romero Diaz CE (2012) The Racial Microaggressions Scale (RMAS): a new scale to measure experiences of racial microaggressions in people of color. Cult Divers Ethnic Minor Psychol 18(2):153–164
Waelde LC, Pennington D, Mahan C, Mahan R, Kabour M, Marquett R (2010) Psychometric properties of the Race-Related Events Scale. Psychol Trauma Theory Res Pract Policy 2(1):4–11
Bird ST, Bogart LM (2001) Perceived race-based and socioeconomic status(SES)-based discrimination in interactions with health care providers. Ethn Dis 11(3):554–563
Hausmann LR, Kressin NR, Hanusa BH, Ibrahim SA (2010) Perceived racial discrimination in health care and its association with patients’ healthcare experiences: does the measure matter? Ethn Dis 20(1):40–47
Peek ME, Nunez-Smith M, Drum M, Lewis TT (2011) Adapting the everyday discrimination scale to medical settings: reliability and validity testing in a sample of African American patients. Ethn Dis 21(4):502–509
Kershaw KN, Diez Roux AV, Burgard SA, Lisabeth LD, Mujahid MS, Schulz AJ (2011) Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol 174(5):537–545
Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV (2015) Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation 131(2):141–148
Alson JG, Robinson WR, Pittman L, Doll KM (2021) Incorporating measures of structural racism into population studies of reproductive health in the United States: a narrative review. Health Equity 5(1):49–58
Groos M, Wallace M, Hardeman R, Theall KP (2018) Measuring inequity: a systematic review of methods used to quantify structural racism. J Health Disparities Res Pract 11(2):13
Adkins-Jackson PB, Chantarat T, Bailey ZD, Ponce NA (2022) Measuring structural racism: a guide for epidemiologists and other health researchers. Am J Epidemiol 191(4):539–547
Jahn JL (2021) Invited commentary: comparing approaches to measuring structural racism. Am J Epidemiol 191(4):548–551
Dean LT, Thorpe RJ, Jr (2022) What structural racism is (or is not) and how to measure it: clarity for public health and medical researchers. Am J Epidemiol
Dougherty GB, Golden SH, Gross AL, Colantuoni E, Dean LT (2020) Measuring structural racism and its association with BMI. Am J Prev Med 59(4):530–537
James P, Hart JE, Hipp JA, Mitchell JA, Kerr J, Hurvitz PM, Glanz K, Laden F (2017) GPS-based exposure to greenness and walkability and accelerometry-based physical activity. Cancer Epidemiol Biomark Prev 26(4):525–532
Sweeney MR, Nichols HB, Jones RR, Olshan AF, Keil AP, Engel LS, James P, Jackson CL, Sandler DP, White AJ (2022) Light at night and the risk of breast cancer: findings from the Sister study. Environ Int 169:107495
Iyer HS, Valeri L, James P, Chen JT, Hart JE, Laden F, Holmes MD, Rebbeck TR (2020) The contribution of residential greenness to mortality among men with prostate cancer: a registry-based cohort study of Black and White men. Environ Epidemiol 4(2):e087
Health NCfE (2011) Impact of the built environment on health. In: Healthy community design. vol 2022. Centers for Disease Control
Berkovitz C (2020) Environmental racism has left black communities especially vulnerable to COVID-19. In: Rights & justice, vol 2022. The Century Foundation
Poisonous Homes: The Fight for Environmental Justice in Federally Assisted Housing. https://www.povertylaw.org/wp-content/uploads/2020/06/environmental_justice_report_final-rev2.pdf
Ashing KT, Jones V, Bedell F, Phillips T, Erhunmwunsee L (2022) Calling attention to the role of race-driven societal determinants of health on aggressive tumor biology: a focus on Black Americans. JCO Oncol Pract 18(1):15–22
Naess O, Piro FN, Nafstad P, Smith GD, Leyland AH (2007) Air pollution, social deprivation, and mortality: a multilevel cohort study. Epidemiology 18(6):686–694
Jbaily A, Zhou X, Liu J, Lee TH, Kamareddine L, Verguet S, Dominici F (2022) Air pollution exposure disparities across US population and income groups. Nature 601(7892):228–233
Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, Post WS, Kaufman JD, Navas-Acien A (2014) Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health 104(11):2130–2137
Schulz AJ, Omari A, Ward M, Mentz GB, Demajo R, Sampson N, Israel BA, Reyes AG, Wilkins D (2020) Independent and joint contributions of economic, social and physical environmental characteristics to mortality in the Detroit Metropolitan Area: a study of cumulative effects and pathways. Health Place 65:102391
James W, Jia C, Kedia S (2012) Uneven magnitude of disparities in cancer risks from air toxics. Int J Environ Res Public Health 9(12):4365–4385
Turner MC, Andersen ZJ, Baccarelli A, Diver WR, Gapstur SM, Pope CA 3rd, Prada D, Samet J, Thurston G, Cohen A (2020) Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin 70(6):460–479
Huang CC, Ho CH, Chen YC, Hsu CC, Lin HJ, Tian YF, Wang JJ, Guo HR (2020) Impact of carbon monoxide poisoning on the risk of breast cancer. Sci Rep 10(1):20450
Kazemiparkouhi F, Eum KD, Wang B, Manjourides J, Suh HH (2020) Long-term ozone exposures and cause-specific mortality in a US Medicare cohort. J Expo Sci Environ Epidemiol 30(4):650–658
Eum KD, Kazemiparkouhi F, Wang B, Manjourides J, Pun V, Pavlu V, Suh H (2019) Long-term NO2 exposures and cause-specific mortality in American older adults. Environ Int 124:10–15
Santibanez-Andrade M, Quezada-Maldonado EM, Osornio-Vargas A, Sanchez-Perez Y, Garcia-Cuellar CM (2017) Air pollution and genomic instability: the role of particulate matter in lung carcinogenesis. Environ Pollut 229:412–422
Pritchett N, Spangler EC, Gray GM, Livinski AA, Sampson JN, Dawsey SM, Jones RR (2022) Exposure to outdoor particulate matter air pollution and risk of gastrointestinal cancers in adults: a systematic review and meta-analysis of epidemiologic evidence. Environ Health Perspect 130(3):36001
Youogo LMK, Parent ME, Hystad P, Villeneuve PJ (2022) Ambient air pollution and prostate cancer risk in a population-based Canadian case-control study. Environ Epidemiol 6(4):e219
Chen J, Rodopoulou S, Strak M, de Hoogh K, Taj T, Poulsen AH, Andersen ZJ, Bellander T, Brandt J, Zitt E et al (2022) Long-term exposure to ambient air pollution and bladder cancer incidence in a pooled European cohort: the ELAPSE project. Br J Cancer 126(10):1499–1507
Yeh HL, Hsu SW, Chang YC, Chan TC, Tsou HC, Chang YC, Chiang PH (2017) Spatial analysis of ambient PM2.5 exposure and bladder cancer mortality in Taiwan. Int J Environ Res Public Health 14(5):508
Zare Sakhvidi MJ, Lequy E, Goldberg M, Jacquemin B (2020) Air pollution exposure and bladder, kidney and urinary tract cancer risk: a systematic review. Environ Pollut 267:115328
Hwang J, Bae H, Choi S, Yi H, Ko B, Kim N (2020) Impact of air pollution on breast cancer incidence and mortality: a nationwide analysis in South Korea. Sci Rep 10(1):5392
Andersen ZJ, Stafoggia M, Weinmayr G, Pedersen M, Galassi C, Jorgensen JT, Oudin A, Forsberg B, Olsson D, Oftedal B et al (2017) Long-term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 european cohorts within the ESCAPE project. Environ Health Perspect 125(10):107005
Andersen ZJ, Ravnskjaer L, Andersen KK, Loft S, Brandt J, Becker T, Ketzel M, Hertel O, Lynge E, Brauner EV (2017) Long-term exposure to fine particulate matter and breast cancer incidence in the Danish Nurse Cohort Study. Cancer Epidemiol Biomark Prev 26(3):428–430
Hart JE, Bertrand KA, DuPre N, James P, Vieira VM, Tamimi RM, Laden F (2016) Long-term particulate matter exposures during adulthood and risk of breast cancer incidence in the nurses’ health study ii prospective cohort. Cancer Epidemiol Biomark Prev 25(8):1274–1276
Prada D, Baccarelli AA, Terry MB, Valdez L, Cabrera P, Just A, Kloog I, Caro H, Garcia-Cuellar C, Sanchez-Perez Y et al (2021) Long-term PM2.5 exposure before diagnosis is associated with worse outcome in breast cancer. Breast Cancer Res Treat 188(2):525–533
Villanueva C, Chang J, Ziogas A, Bristow RE, Vieira VM (2021) Ambient air pollution and ovarian cancer survival in California. Gynecol Oncol 163(1):155–161
Arias-Perez RD, Taborda NA, Gomez DM, Narvaez JF, Porras J, Hernandez JC (2020) Inflammatory effects of particulate matter air pollution. Environ Sci Pollut Res Int 27(34):42390–42404
Li R, Zhou R, Zhang J (2018) Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett 15(5):7506–7514
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E (2020) Environmental and health impacts of air pollution: a review. Front Public Health 8:14
Peluso M, Ceppi M, Munnia A, Puntoni R, Parodi S (2001) Analysis of 13 (32)P-DNA postlabeling studies on occupational cohorts exposed to air pollution. Am J Epidemiol 153(6):546–558
Dunn BP (1991) Carcinogen adducts as an indicator for the public health risks of consuming carcinogen-exposed fish and shellfish. Environ Health Perspect 90:111–116
Peluso M, Airoldi L, Armelle M, Martone T, Coda R, Malaveille C, Giacomelli G, Terrone C, Casetta G, Vineis P (1998) White blood cell DNA adducts, smoking, and NAT2 and GSTM1 genotypes in bladder cancer: a case-control study. Cancer Epidemiol Biomark Prev 7(4):341–346
Deben C, Van den Bossche J, Van Der Steen N, Lardon F, Wouters A, de Beeck KO, Hermans C, Jacobs J, Peeters M, Van Camp G et al (2017) Deep sequencing of the TP53 gene reveals a potential risk allele for non-small cell lung cancer and supports the negative prognostic value of TP53 variants. Tumour Biol 39(2):1010428317694327
Zhou W, Tian D, He J, Wang Y, Zhang L, Cui L, Jia L, Zhang L, Li L, Shu Y et al (2016) Repeated PM2.5 exposure inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation. Oncotarget 7(15):20691–20703
Yu XJ, Yang MJ, Zhou B, Wang GZ, Huang YC, Wu LC, Cheng X, Wen ZS, Huang JY, Zhang YD et al (2015) Characterization of somatic mutations in air pollution-related lung cancer. EBioMedicine 2(6):583–590
Jiang CL, He SW, Zhang YD, Duan HX, Huang T, Huang YC, Li GF, Wang P, Ma LJ, Zhou GB et al (2017) Air pollution and DNA methylation alterations in lung cancer: a systematic and comparative study. Oncotarget 8(1):1369–1391
Letellier N, Wing SE, Yang JA, Gray SW, Benmarhnia T, Erhunmwunsee L, Jankowska MM (2022) The role of neighborhood air pollution exposure on somatic non-small cell lung cancer mutations in the Los Angeles Basin (2013–2018). Int J Environ Res Public Health 19(17):11027
Wang Z, Zhai Z, Chen C, Tian X, Xing Z, Xing P, Yang Y, Zhang J, Wang C, Dong L (2022) Air pollution particles hijack peroxidasin to disrupt immunosurveillance and promote lung cancer. Elife 11:e75345
Dobreva ZG, Kostadinova GS, Popov BN, Petkov GS, Stanilova SA (2015) Proinflammatory and anti-inflammatory cytokines in adolescents from Southeast Bulgarian cities with different levels of air pollution. Toxicol Ind Health 31(12):1210–1217
Niu BY, Li WK, Li JS, Hong QH, Khodahemmati S, Gao JF, Zhou ZX (2020) Effects of DNA damage and oxidative stress in human bronchial epithelial cells exposed to PM2.5 from Beijing, China, in Winter. Int J Environ Res Public Health 17(13):4874
Reyes-Caballero H, Rao X, Sun Q, Warmoes MO, Lin P, Sussan TE, Park B, Fan TW, Maiseyeu A, Rajagopalan S et al (2020) Author correction: air pollution-derived particulate matter dysregulates hepatic Krebs cycle, glucose and lipid metabolism in mice. Sci Rep 10(1):5082
Conroy SM, Shariff-Marco S, Koo J, Yang J, Keegan TH, Sangaramoorthy M, Hertz A, Nelson DO, Cockburn M, Satariano WA et al (2017) Racial/ethnic differences in the impact of neighborhood social and built environment on breast cancer risk: the neighborhoods and breast cancer study. Cancer Epidemiol Biomark Prev 26(4):541–552
Mayhand KN, Handorf EA, Ortiz AG, Gonzalez ET, Devlin A, Sorice KA, Esnaola N, Fisher S, Lynch SM (2021) Effect of neighborhood and individual-level socioeconomic factors on colorectal cancer screening adherence. Int J Environ Res Public Health 18(9):4398
Rosenzweig MQ, Althouse AD, Sabik L, Arnold R, Chu E, Smith TJ, Smith K, White D, Schenker Y (2021) The association between area deprivation index and patient-reported outcomes in patients with advanced cancer. Health Equity 5(1):8–16
DeRouen MC, Yang J, Jain J, Weden MM, Gomez SL, Shariff-Marco S (2022) Disparities in prostate cancer survival according to neighborhood archetypes, a population-based study. Urology 163:138–147
Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, Nelson DO, Schupp CW, Shema SJ, Cockburn M et al (2014) Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomark Prev 23(5):793–811
Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, Satariano WA, Glaser SL (2015) The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer 121(14):2314–2330
Aviv A, Anderson JJ, Shay JW (2017) Mutations, cancer and the telomere length paradox. Trends Cancer 3(4):253–258
Powell-Wiley TM, Gebreab SY, Claudel SE, Ayers C, Andrews MR, Adu-Brimpong J, Berrigan D, Davis SK (2020) The relationship between neighborhood socioeconomic deprivation and telomere length: the 1999–2002 National Health and Nutrition Examination Survey. SSM Popul Health 10:100517
Zhu X, Han W, Xue W, Zou Y, Xie C, Du J, Jin G (2016) The association between telomere length and cancer risk in population studies. Sci Rep 6:22243
Urquidi V, Tarin D, Goodison S (1998) Telomerase in cancer: clinical applications. Ann Med 30(5):419–430
Brown R, Hailu EM, Needham BL, Roux AD, Seeman TE, Lin J, Mujahid MS (2021) Neighborhood social environment and changes in leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis (MESA). Health Place 67:102488
Alexeeff SE, Schaefer CA, Kvale MN, Shan J, Blackburn EH, Risch N, Ranatunga DK, Jorgenson E, Hoffmann TJ, Sakoda LC et al (2019) Telomere length and socioeconomic status at neighborhood and individual levels among 80,000 adults in the Genetic Epidemiology Research on Adult Health and Aging cohort. Environ Epidemiol 3(3):e049
Coimbra BM, Carvalho CM, van Zuiden M, Williamson RE, Ota VK, Mello AF, Belangero SI, Olff M, Mello MF (2022) The impact of neighborhood context on telomere length: a systematic review. Health Place 74:102746
Barber LE, Zirpoli GR, Cozier YC, Rosenberg L, Petrick JL, Bertrand KA, Palmer JR (2021) Neighborhood disadvantage and individual-level life stressors in relation to breast cancer incidence in US Black women. Breast Cancer Res 23(1):108
Basudhar D, Glynn SA, Greer M, Somasundaram V, No JH, Scheiblin DA, Garrido P, Heinz WF, Ryan AE, Weiss JM et al (2017) Coexpression of NOS2 and COX2 accelerates tumor growth and reduces survival in estrogen receptor-negative breast cancer. Proc Natl Acad Sci USA 114(49):13030–13035
Guidi J, Lucente M, Sonino N, Fava GA (2021) Allostatic load and its impact on health: a systematic review. Psychother Psychosom 90(1):11–27
Zhao H, Song R, Ye Y, Chow WH, Shen J (2021) Allostatic score and its associations with demographics, healthy behaviors, tumor characteristics, and mitochondrial DNA among breast cancer patients. Breast Cancer Res Treat 187(2):587–596
Costanzo ES, Sood AK, Lutgendorf SK (2011) Biobehavioral influences on cancer progression. Immunol Allergy Clin N Am 31(1):109–132
Nazmi A, Diez Roux A, Ranjit N, Seeman TE, Jenny NS (2010) Cross-sectional and longitudinal associations of neighborhood characteristics with inflammatory markers: findings from the multi-ethnic study of atherosclerosis. Health Place 16(6):1104–1112
Iyer HS, Hart JE, James P, Elliott EG, DeVille NV, Holmes MD, De Vivo I, Mucci LA, Laden F, Rebbeck TR (2022) Impact of neighborhood socioeconomic status, income segregation, and greenness on blood biomarkers of inflammation. Environ Int 162:107164
Nagar SD, Conley AB, Sharma S, Rishishwar L, Jordan IK, Marino-Ramirez L (2021) Comparing genetic and socioenvironmental contributions to ethnic differences in C-reactive protein. Front Genet 12:738485
Muscatell KA, Brosso SN, Humphreys KL (2020) Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry 25(9):2189–2199
Liu RS, Aiello AE, Mensah FK, Gasser CE, Rueb K, Cordell B, Juonala M, Wake M, Burgner DP (2017) Socioeconomic status in childhood and C reactive protein in adulthood: a systematic review and meta-analysis. J Epidemiol Community Health 71(8):817–826
Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Zimmerman MB, Penedo FJ, Lucci JA 3rd, Ganjei-Azar P et al (2012) Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol 30(23):2885–2890
Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I (2006) Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol 24(7):1105–1111
Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ (2013) Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast Cancer Res Treat 137(1):261–271
Umberson D (1987) Family status and health behaviors: social control as a dimension of social integration. J Health Soc Behav 28(3):306–319
Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC (2016) Breast cancer and social environment: getting by with a little help from our friends. Breast Cancer Res 18(1):54
Brisson D (2014) Neighborhood social cohesion. Oxford-Bibliographics in Social Work
Rosenblatt AM, Crews DC, Powe NR, Zonderman AB, Evans MK, Tuot DS (2021) Association between neighborhood social cohesion, awareness of chronic diseases, and participation in healthy behaviors in a community cohort. BMC Public Health 21(1):1611
Dustin T, Duncan IK (2014) Neighborhoods and health, 2nd edn. Oxford University Press, Oxford
Martin AL, Brown RE (2010) The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res 207(1):196–207
Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS (2007) Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32(8–10):966–980
Olsson IAS, Westlund K (2007) More than numbers matter: the effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl Anim Behav Sci 103(3):229–254
Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, Li X, Li Y, Xiong W, Li G et al (2020) Chronic stress promotes cancer development. Front Oncol 10:1492
Antoni MH, Bouchard LC, Jacobs JM, Lechner SC, Jutagir DR, Gudenkauf LM, Carver CS, Lutgendorf S, Cole SW, Lippman M et al (2016) Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: an exploratory analysis. Psychoneuroendocrinology 74:269–277
Ross K (2008) Mapping pathways from stress to cancer progression. J Natl Cancer Inst 100(13):914–915
Kubzansky LD, Seeman TE, Glymour MM (2014) Biological pathways linking social conditions and health: plausible mechanisms and emerging puzzles. In: Berkman LF, Kawachi I, Glymour MM (eds) Social epidemiology. Oxford University Press, Oxford
Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT (2006) Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA 103(45):17058–17063
Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW (2006) Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 12(2):369–375
Xia N, Li H (2018) Loneliness, social isolation, and cardiovascular health. Antioxid Redox Signal 28(9):837–851
Carter SE, Ong ML, Simons RL, Gibbons FX, Lei MK, Beach SRH (2019) The effect of early discrimination on accelerated aging among African Americans. Health Psychol 38(11):1010–1013
Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, Blackburn EH, Thomas SB (2016) Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology 63:10–16
Brody GH, Miller GE, Yu T, Beach SR, Chen E (2016) Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: a replication across two longitudinal cohorts. Psychol Sci 27(4):530–541
Hailu EM, Lewis TT, Needham BL, Lin J, Seeman TE, Mujahid MS (2021) Longitudinal associations between discrimination, neighborhood social cohesion, and telomere length: the multi-ethnic study of atherosclerosis (MESA). J Gerontol A Biol Sci Med Sci
Krieger N, Jahn JL, Waterman PD (2017) Jim Crow and estrogen-receptor-negative breast cancer: US-born black and white non-Hispanic women, 1992–2012. Cancer Causes Control 28(1):49–59
Perry LM, Walsh LE, Horswell R, Miele L, Chu S, Melancon B, Lefante J, Blais CM, Rogers JL, Hoerger M (2021) Racial disparities in end-of-life care between black and white adults with metastatic cancer. J Pain Symptom Manag 61(2):342–349
Penner LA, Harper FWK, Dovidio JF, Albrecht TL, Hamel LM, Senft N, Eggly S (2017) The impact of Black cancer patients’ race-related beliefs and attitudes on racially-discordant oncology interactions: a field study. Soc Sci Med 191:99–108
Penner LA, Dovidio JF, Hagiwara N, Foster T, Albrecht TL, Chapman RA, Eggly S (2016) An analysis of race-related attitudes and beliefs in Black cancer patients: implications for health care disparities. J Health Care Poor Underserved 27(3):1503–1520
Manning M, Lucas T, Purrington K, Thompson H, Albrecht TL, Penner L (2022) Moderators of the effects of perceived racism and discrimination on cancer-related health behaviors among two samples of African Americans. Soc Sci Med 114982
Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Levy DJ, Kemeny M, Brodish AB, Malanchuk O et al (2015) Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: a 20-year prospective study. Psychoneuroendocrinology 62:279–291
Cuevas AG, Reitzel LR, Adams CE, Cao Y, Nguyen N, Wetter DW, Watkins KL, Regan SD, McNeill LH (2014) Discrimination, affect, and cancer risk factors among African Americans. Am J Health Behav 38(1):31–41
Forde AT, Sims M, Wang X, Barber S, Diez Roux AV (2021) The role of perceived discrimination in predicting changes in health behaviours among African Americans in the Jackson Heart Study. J Epidemiol Community Health 75(12):1222–1231
Pantesco EJ, Leibel DK, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, Weng NP, Evans MK, Zonderman AB, Beatty Moody DL (2018) Multiple forms of discrimination, social status, and telomere length: interactions within race. Psychoneuroendocrinology 98:119–126
Liu SY, Kawachi I (2017) Discrimination and telomere length among older adults in the United States. Public Health Rep 132(2):220–230
Kershaw KN, Lewis TT, Diez Roux AV, Jenny NS, Liu K, Penedo FJ, Carnethon MR (2016) Self-reported experiences of discrimination and inflammation among men and women: the multi-ethnic study of atherosclerosis. Health Psychol 35(4):343–350
Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, Berkman LF (2012) Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc Sci Med 75(5):922–931
Cobb RJ, Parker LJ, Thorpe RJ (2020) Self-reported instances of major discrimination, race/ethnicity, and inflammation among older adults: evidence from the health and retirement study. J Gerontol A Biol Sci Med Sci 75(2):291–296
Forde AT, Sims M, Muntner P, Lewis T, Onwuka A, Moore K, Diez Roux AV (2020) Discrimination and hypertension risk among African Americans in the Jackson Heart Study. Hypertension 76(3):715–723
Forde AT, Lewis TT, Kershaw KN, Bellamy SL, Diez Roux AV (2021) Perceived discrimination and hypertension risk among participants in the multi-ethnic study of atherosclerosis. J Am Heart Assoc 10(5):e019541
Geronimus AT (1992) The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis 2(3):207–221
Forde AT, Crookes DM, Suglia SF, Demmer RT (2019) The weathering hypothesis as an explanation for racial disparities in health: a systematic review. Ann Epidemiol 33(1–18):e13
Geronimus AT, Hicken M, Keene D, Bound J (2006) “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 96(5):826–833
Bird CE, Seeman T, Escarce JJ, Basurto-Davila R, Finch BK, Dubowitz T, Heron M, Hale L, Merkin SS, Weden M et al (2010) Neighbourhood socioeconomic status and biological “wear and tear” in a nationally representative sample of US adults. J Epidemiol Community Health 64(10):860–865
Figueroa JF, Frakt AB, Jha AK (2020) Addressing social determinants of health: time for a polysocial risk score. JAMA 323(16):1553–1554
Kinner SA, Borschmann R (2020) Polysocial risk scores for assessing social determinants of health. JAMA 324(16):1680–1681
Javed Z, Valero-Elizondo J, Dudum R, Khan SU, Dubey P, Hyder AA, Xu J, Bilal U, Kash BA, Cainzos-Achirica M et al (2021) Development and validation of a polysocial risk score for atherosclerotic cardiovascular disease. Am J Prev Cardiol 8:100251
Figueroa JF, Jha AK (2020) Polysocial risk scores for assessing social determinants of health-reply. JAMA 324(16):1681–1682
Zhang H, Hu H, Diller M, Hogan WR, Prosperi M, Guo Y, Bian J (2021) Semantic standards of external exposome data. Environ Res 197:111185
Vineis P, Robinson O, Chadeau-Hyam M, Dehghan A, Mudway I, Dagnino S (2020) What is new in the exposome? Environ Int 143:105887
Hu H, Zhao J, Savitz DA, Prosperi M, Zheng Y, Pearson TA (2020) An external exposome-wide association study of hypertensive disorders of pregnancy. Environ Int 141:105797
Acknowledgments
We thank our funders, National Cancer Institute Intramural Research Program, Center for Cancer Research, and the Cancer Prevention Fellowship Program for their support in completing this work.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (NCI), Center for Cancer Research; Brittany Lord and Alexandra Harris are supported by the Cancer Prevention Fellowship Program.
Author information
Authors and Affiliations
Contributions
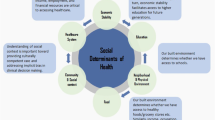
All authors contributed to the conception and design of the manuscript. The first draft of the manuscript was written by BL and A. H. B.L. prepared Fig. 1. Manuscript revisions and figure edits were completed by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lord, B.D., Harris, A.R. & Ambs, S. The impact of social and environmental factors on cancer biology in Black Americans. Cancer Causes Control 34, 191–203 (2023). https://doi.org/10.1007/s10552-022-01664-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-022-01664-w