Abstract
Purpose
The interim analysis of the phase IIIb LUCY trial demonstrated the clinical effectiveness of olaparib in patients with germline BRCA-mutated (gBRCAm), human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (mBC), with median progression-free survival (PFS) of 8.11 months, which was similar to that in the olaparib arm of the phase III OlympiAD trial (7.03 months). This prespecified analysis provides final overall survival (OS) and safety data.
Methods
The open-label, single-arm LUCY trial of olaparib (300 mg, twice daily) enrolled adults with gBRCAm or somatic BRCA-mutated (sBRCAm), HER2-negative mBC. Patients had previously received a taxane or anthracycline for neoadjuvant/adjuvant or metastatic disease and up to two lines of chemotherapy for mBC.
Results
Of 563 patients screened, 256 (gBRCAm, n = 253; sBRCAm, n = 3) were enrolled. In the gBRCAm cohort, median investigator-assessed PFS (primary endpoint) was 8.18 months and median OS was 24.94 months. Olaparib was clinically effective in all prespecified subgroups: hormone receptor status, previous chemotherapy for mBC, previous platinum-based chemotherapy (including by line of therapy), and previous cyclin-dependent kinase 4/6 inhibitor use. The most frequent treatment-emergent adverse events (TEAEs) were nausea (55.3%) and anemia (39.2%). Few patients (6.3%) discontinued olaparib owing to a TEAE. No deaths associated with AEs occurred during the study treatment or 30-day follow-up.
Conclusion
The LUCY patient population reflects a real-world population in line with the licensed indication of olaparib in mBC. These findings support the clinical effectiveness and safety of olaparib in patients with gBRCAm, HER2-negative mBC.
Clinical trial registration
Clinical trials registration number: NCT03286842
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss-of-function mutations in the breast cancer (BC) susceptibility genes 1 and 2 (BRCA1 and BRCA2; BRCA) are associated with an increased risk of developing BC [1, 2]. Patients with a germline BRCA mutation are often young at initial BC diagnosis and present with aggressive disease [3]. Germline BRCA mutations have been detected in approximately 9.7% of patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic BC (mBC), with prevalence being higher in patients with triple-negative BC (TNBC; 10–20%) than in those with hormone receptor (HR)-positive BC (2–8%) [4,5,6]. However, with the relative prevalence of the two subtypes, the majority of patients with germline BRCA-mutated (gBRCAm) BC are HR-positive. Due to the hereditary component of gBRCAm BC, the prevalence is higher at approximately 23% in those with a family history of BC or ovarian cancer [5].
The BRCA1 and BRCA2 proteins play critical roles in DNA damage response pathways, particularly in the repair of DNA double-strand breaks [7]. Tumor cells lacking functional BRCA1 or BRCA2 proteins show increased sensitivity to DNA-damaging agents, including poly(ADP-ribose) polymerase (PARP) enzyme inhibitors, as well as platinum-based and non-platinum-based chemotherapies [8,9,10,11,12].
Two phase III randomized clinical studies have delivered robust evidence regarding the efficacy and safety of PARP inhibitors in patients with gBRCAm, HER2-negative locally advanced and/or mBC: OlympiAD (olaparib versus physician’s choice of chemotherapy in mBC, NCT02000622) and EMBRACA (talazoparib versus physician’s choice of chemotherapy in locally advanced and mBC, NCT01945775) [13,14,15,16,17,18,19]. Subsequently, both PARP inhibitors were approved as targeted treatments for patients with gBRCAm, HER2-negative mBC (and locally advanced BC in Europe; talazoparib is also approved for locally advanced BC in the USA) who have previously been treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting [15, 20,21,22,23].
In the OlympiA study (NCT02032823), 1 year of adjuvant olaparib treatment after completion of neoadjuvant or adjuvant chemotherapy and local treatment resulted in significantly longer invasive and distant disease-free survival and fewer deaths compared with placebo in patients with gBRCAm, HER2-negative, high-risk early BC [24, 25]. Based on these findings, olaparib was approved as an adjuvant treatment for patients with gBRCAm, HER2-negative, high-risk early BC who have previously been treated with neoadjuvant or adjuvant chemotherapy [21, 26].
The phase IIIb LUCY trial (NCT03286842) has further demonstrated the clinical effectiveness and well-tolerated safety profile of olaparib in patients with gBRCAm, HER2‑negative mBC, in a setting designed to reflect routine clinical practice more closely than the OlympiAD trial [15, 27]. Encouraging data have suggested that patients with a somatic BRCA mutation (sBRCAm) may benefit from PARP inhibition in the ovarian cancer, prostate cancer, and mBC settings [28,29,30,31,32]. Accordingly, the LUCY trial also permitted enrollment of patients with a sBRCAm [27]. At the data cutoff for the prespecified interim analysis (September 23, 2019), median progression-free survival (PFS; 8.11 months) was consistent with that reported for olaparib in the OlympiAD trial (7.03 months), and no new safety signals were observed [15, 27]. This final planned analysis (data cutoff September 1, 2021) includes assessments of overall survival (OS) and safety.
Patients and methods
Study design and treatment
Details of the LUCY trial have been reported previously [27]. In brief, this open-label, single-arm, multicenter, international, phase IIIb study enrolled adults with a gBRCAm or sBRCAm and HER2-negative mBC (triple-negative or HR-positive). Patients with a sBRCAm were permitted following a study protocol amendment (April 27, 2018). Eligible patients had received a maximum of two lines of prior chemotherapy for mBC and either taxane- or anthracycline-based chemotherapy in any setting. Patients treated with prior platinum-based chemotherapy and patients with stable brain metastases were eligible. Those with HR-positive mBC who had previously completed at least one line of endocrine therapy in either an adjuvant or metastatic setting and were considered unsuitable for further endocrine therapy were eligible.
Patients received olaparib tablets (300 mg twice daily) until disease progression, unacceptable toxicity, or other protocol-specified discontinuation criteria were met. Patients who discontinued study treatment were followed to record progression (if treatment was discontinued in the absence of progression), use of subsequent anti-cancer therapies, time to second progression or death (PFS2), and OS.
Study outcomes and assessments
Tumor assessments were performed at each study visit, up to the first instance of disease progression, and then in accordance with local practice. The primary outcome was investigator-assessed PFS in the gBRCAm cohort, defined as the time from first dose of olaparib to the date of progression or death from any cause. Physician-defined clinical response could be radiologic (as per Response evaluation criteria in solid tumors [RECIST] version 1.1) or symptomatic, or clear progression of non-measurable disease, if progression could be documented.
Secondary clinical effectiveness outcomes (assessed in the gBRCAm cohort) were: OS (time from first dose of olaparib to the date of death from any cause); time to first subsequent treatment or death (TFST; time from first dose of olaparib to first subsequent treatment commencement or death); time to study treatment discontinuation or death (TDT); time to second subsequent treatment or death (TSST); PFS2 (time from first dose of olaparib to the earliest progression event after the event used for the primary endpoint or death from any cause); clinical response rate (CRR); and duration of clinical response (DoCR; time from when the investigator first assessed the patient as responding to the date of progression or death from any cause, in the absence of progression). Time to onset of a clinical response in patients in the gBRCAm cohort was assessed in a post hoc analysis. Clinical effectiveness outcomes assessed in the sBRCAm cohort were exploratory.
Tolerability and safety were secondary outcomes. Actual treatment duration was calculated by considering the duration of dose interruptions. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and coded to preferred terms using Medical Dictionary for Regulatory Activities version 22.1. Treatment-emergent AEs (TEAEs) were defined as those with an onset date or a pre-existing AE worsening following the first dose of study treatment through to 30 days after the last dose of study treatment. Prespecified AEs of special interest (AESI) for olaparib were myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), a new primary malignancy (other than MDS/AML), and pneumonitis.
Statistical analyses
The primary and secondary clinical effectiveness outcomes are reported for all patients in the gBRCAm cohort who received at least one dose of olaparib. Safety outcomes are summarized for all patients (gBRCAm and sBRCAm) who received at least one dose of olaparib. The final analysis was planned after reaching at least 130 OS events (approximately 52% data maturity) in the gBRCAm cohort. The sample size estimate for OS was based on recruitment of 250 patients with a germline BRCA mutation; if median OS was 19 months and analyzed after 130 OS events, the 95% confidence interval (CI) for the median would be predicted to extend from 16.0 to 22.6 months (based on the formula of Collett) [33].
The Kaplan–Meier method was used to generate survival curves for all time-to-event endpoints (PFS, OS, DoCR, TFST, TSST, TDT, and PFS2), from which estimates of the median were calculated, together with event rates at 6-monthly intervals and their associated 95% CIs. The associated 95% CI for the median was derived based on the Brookmeyer–Crowley method. A 95% CI for CRR was calculated using the Clopper–Pearson exact method for binomial proportions. The median duration of follow-up was determined in patients who were censored.
Prespecified PFS, OS, and CRR subgroup analyses were performed in the gBRCAm cohort according to HR status (HR-positive or TNBC), previous chemotherapy for mBC (yes or no), previous platinum-based chemotherapy for BC (yes or no; the yes category was further classified into neoadjuvant/adjuvant and metastatic), line of therapy (first-line [i.e. no prior chemotherapy in the first-line advanced/metastatic setting but prior chemotherapy in the neoadjuvant/adjuvant setting] versus second line or later [i.e. prior chemotherapy in the first-line advanced-metastatic setting, with or without prior chemotherapy in neoadjuvant/adjuvant setting]) and prior platinum-based chemotherapy (yes or no), and previous cyclin-dependent kinase 4/6 (CDK4/6) inhibitor treatment for BC (yes or no). Previous chemotherapy for mBC was defined as having received at least one but not more than two lines of chemotherapy in the metastatic setting and did not include chemotherapy given in the neoadjuvant/adjuvant setting; previous endocrine therapy that may have been received by some patients for mBC was not taken into consideration. No formal statistical comparisons were performed among subgroups.
Results
Patient disposition
Between January 17, 2018, and March 21, 2019, 563 patients were screened, and 256 patients were enrolled (gBRCAm, n = 253; sBRCAm, n = 3) (Supplementary Fig. 1). One patient in the gBRCAm cohort did not receive olaparib and was excluded from the full analysis set (n = 255). Fewer patients were enrolled into the sBRCAm cohort (n = 3) than planned (n = 20) owing to the short period of time between the protocol amendment that allowed their inclusion and trial completion. At the data cutoff for this final prespecified analysis, 29 (11.5%) patients were still receiving study treatment. The most common reason for discontinuation of olaparib was disease progression (n = 192; 75.3%).
Baseline characteristics
Baseline characteristics for all patients (gBRCAm and sBRCAm; Table 1) were similar to those for the gBRCAm cohort (Supplementary Table 1). Median age of all patients was 45.0 years (range, 22–75 years) and most patients were White (n = 177 [69.4%]) (Table 1). Slightly more patients (n = 138 [54.1%]) had a BRCA1 mutation only compared with the 109 patients (42.7%) with a BRCA2 mutation only; five patients (2.0%) had both BRCA1 and BRCA2 mutations. Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 (n = 185 [72.5%]) and were initially diagnosed with stage I─II disease according to the American Joint Committee on Cancer (n = 135 [52.9%]). The study population was well balanced with regard to HR status. Eleven patients (4.3%) had central nervous system metastases at baseline. In total, 221 patients (86.7%) had previously received an anthracycline and 226 patients (88.6%) had previously received a taxane in any setting; 192 patients (75.3%) had received both an anthracycline and a taxane. Eighty-one patients (31.8%) received previous platinum-based chemotherapy. In total, 48 patients (59.3%) who had received previous platinum-based chemotherapy had TNBC; none of the patients in the sBRCAm cohort had previously received platinum-based chemotherapy. In this study, 117 patients (45.9%; gBRCAm, n = 116; sBRCAm, n = 1) had previously received chemotherapy for mBC in the first-line setting and 138 patients (54.1%; gBRCAm, n = 136; sBRCAm, n = 2) had not received chemotherapy in the mBC setting.
Clinical effectiveness
At data cutoff, there were 207 PFS events in the gBRCAm cohort (82.1% maturity). The primary endpoint of median PFS was 8.18 months (95% CI 6.97–9.17; Fig. 1a). There were 140 OS events in the gBRCAm cohort (55.6% maturity). Median OS was 24.94 months (95% CI 21.06–28.91 months). (Fig. 1b).
Kaplan–Meier estimates for a PFS and b OS in the gBRCAm cohort (N = 252). Vertical gray dashed lines and corresponding percentages represent the estimated event rates at 12, 24, and 30 months after starting olaparib treatment. BRCA BRCA1 and/or BRCA2; CI confidence interval; gBRCAm germline BRCA-mutated; OS overall survival; PFS progression-free survival. aReasons for censoring (n = 45; 17.9%): progression-free at time of analysis (n = 34; 13.5%), lost to follow-up (n = 1; 0.4%), withdrawn consent (n = 7; 2.8%), terminated study for other reason (n = 3; 1.2%). bReasons for censoring (n = 112; 44.4%): still in survival follow-up (n = 79; 31.3%), terminated study before death (n = 33; 13.1% [lost to follow-up (n = 2; 0.8%), withdrawn consent (n = 28; 11.1%), other reasons (n = 3; 1.2%)])
Almost half of the patients in the gBRCAm cohort (n = 125 [49.6%]) had a clinical response (Table 2). Median DoCR was 8.0 months (interquartile range, 4.2–18.6 months). Median time to onset of a clinical response was 2.6 months (range, 0.7–30.4 months). Of those with a clinical response, 99 patients (79.2%) subsequently progressed or died. In the gBRCAm cohort, 91 patients (36.1%) had stable disease and 32 patients (12.7%) had progressive disease as their best clinical response. Four patients (1.6%) did not have an evaluable post-baseline assessment.
Median TDT and TFST were 7.98 months (95% CI 6.90–8.54) and 9.40 months (95% CI 8.61–10.64), respectively in the gBRCAm cohort (Supplementary Table 2). Median PFS2 and TSST were 14.49 months (95% CI 13.17–17.05) and 14.72 months (95% CI 13.50–17.25), respectively.
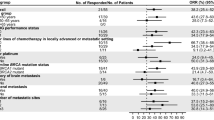
Olaparib was clinically effective across all predefined subgroups of patients in the gBRCAm cohort evaluated. PFS and OS according to HR status, by previous chemotherapy for mBC, prior exposure to platinum-containing therapy (including by line of therapy), and with prior exposure to CDK4/6 inhibitor (HR-positive mBC) are shown in Fig. 2 and Table 3.
Kaplan–Meier estimates for a, b PFS and c, d OS in the gBRCAm cohort by HR status and by previous CT for mBC. BRCA BRCA1 and/or BRCA2; CI confidence interval; CT chemotherapy; gBRCAm germline BRCA-mutated; HR hormone receptor; mBC metastatic breast cancer; OS overall survival; PFS progression-free survival; TNBC triple-negative breast cancer. aYes: received one or two previous lines of CT for metastatic disease (may have also received CT in the neoadjuvant/adjuvant setting). bNo: no previous CT for advanced/metastatic disease but received in the neoadjuvant/adjuvant setting
CRR in the gBRCAm cohort was similar irrespective of HR status, previous chemotherapy for mBC, previous treatment with platinum-based chemotherapy (including by line of therapy), and previous treatment with a CDK4/6 inhibitor (Table 2).
Clinical effectiveness outcomes for the patients in the exploratory sBRCAm cohort are reported in Supplementary File 2.
Safety
In total, 165 patients (79.3%) were still receiving study treatment at the time of progression. The median total treatment duration in all patients was similar to the actual median treatment duration (7.98 months; range, 0.2–43.3 months and 7.46 months; range, 0.1–43.3 months, respectively).
Most patients (n = 246 [96.5%]) experienced a TEAE. The most frequent TEAEs (occurring in at least 20% of all patients) were nausea, anemia, asthenia, vomiting, fatigue, and diarrhea (Fig. 3). Most TEAEs were CTCAE grade 1 or 2 in severity (n = 175 patients [68.6%]); grade 3 or higher TEAEs were reported in 71 patients (27.8%). The most frequent grade 3 or higher TEAEs (reported in at least 2% of patients) were anemia (n = 34 [13.3%]) and neutropenia (n = 16 [6.3%]). Serious TEAEs were reported in 33 patients (12.9%); the most frequent (occurring in more than one patient) were anemia (n = 7 [2.7%]), febrile neutropenia, vomiting, and asthenia (all n = 2 [0.8%]). Overall, 182 (71.4% full analysis set) patients required a dose modification of study treatment; dose modification was due to a TEAE in 111 patients (43.5% full analysis set). The most frequent TEAEs (occurring in at least 20 patients) leading to dose modification were anemia (n = 55 [21.6%]), neutropenia (n = 23 [9.0%]), and vomiting (n = 21 [8.2%]). Few patients discontinued study treatment due to a TEAE (n = 16 [6.3%]).
Most frequent TEAEs (occurring in > 10% patients) (full analysis set, N = 255). Data are reported as number of patients (%) with: grade < 3 TEAEs (black bars), grade ≥ 3 TEAEs (grey bars) and any-grade TEAEs (right-hand side of graph). TEAEs were graded according to Common Terminology Criteria for Adverse Events version 4.0 and coded to preferred terms using the Medical Dictionary for Regulatory Activities version 24.0. TEAE, treatment-emergent adverse event
Ten patients (3.9%) had AESIs: pneumonitis (n = 5) and MDS, bladder cancer in situ (stage 0), neoplasm of the appendix, pancreatic carcinoma, and radiation fibrosis (all n = 1). Following database lock, a review of the patient with radiation fibrosis (originally identified as a pneumonitis AESI) determined that the patient did not have an AESI. All pneumonitis TEAEs were grade 2 or less; two cases led to discontinuation of olaparib.
No deaths associated with AEs were reported during the study treatment period or during the 30-day safety follow-up period following the last dose of olaparib. Two deaths (0.8%) associated with AEs were reported after the 30-day safety follow-up period. One death (0.4%) occurred more than 30 days after the last treatment dose and was related to both the disease under investigation and an AE. Supplementary Table 3 summarizes the most frequent treatment-related AEs.
Discussion
The clinical effectiveness of olaparib in the LUCY trial supports previous findings from the randomized, phase III OlympiAD trial of olaparib versus chemotherapy of physician’s choice in patients with gBRCAm, HER2-negative mBC, underscoring the value of olaparib in this patient population [14, 15].
At the data cutoff for this final prespecified analysis, the median PFS (primary endpoint) in the gBRCAm cohort (8.18 months) was similar to that reported for olaparib in the primary analysis of the OlympiAD trial (7.03 months) [15]. Interestingly, the median OS in the gBRCAm cohort of the LUCY trial (24.94 months) was numerically longer than that reported for olaparib in the final prespecified analysis of OlympiAD (19.25 months) [14]. This difference in survival may reflect the higher proportion of patients in LUCY (~ 54%) compared with OlympiAD (~ 29%) who had not received prior chemotherapy for mBC, which is supported by the longer PFS and OS seen in this subgroup in both studies [14, 15]. However, it should be noted that median OS in patients who had received prior chemotherapy for mBC was also longer in LUCY (22.74 months) than in OlympiAD (18.83 months) [14]. Median total treatment duration and median actual treatment duration were similar in LUCY (8.0 months and 7.5 months, respectively) and OlympiAD (8.2 months and 7.5 months, respectively) [14], suggesting that a difference in exposure to olaparib treatment cannot account for the difference in OS.
The efficacy of olaparib was even more profound in the OlympiA study, where statistically significant improvements in median invasive and distant disease-free survival and OS were observed in patients with HER2-negative, high-risk early BC treated with adjuvant olaparib compared with those who received placebo, further suggesting that earlier treatment with olaparib results in improved efficacy [24, 25]. Targeted treatment would reasonably be expected to offer greatest clinical benefit when there is limited clonal evolution in response to prior treatment [15, 24, 27, 34].
In predefined subgroup analyses, olaparib was clinically effective (as assessed by PFS and OS) in all key subgroups of patients in the gBRCAm cohort, including HR status, previous chemotherapy for mBC, previous platinum-based chemotherapy (including by line of therapy), or previous treatment with CDK4/6 inhibitors. Accordingly, the findings reinforce the clinical efficacy of olaparib in patients with TNBC and HR-positive disease, which is consistent with olaparib targeting the same underlying cause of the disease in these patients. Patients with TNBC and those who received platinum-based chemotherapy had a similar response to olaparib, which was expected owing to the overlap of patient populations in these subgroups. Questions remain regarding the optimal sequencing of treatment with olaparib and CDK4/6 inhibitors. Real-world evidence indicates that patients with gBRCAm who receive CDK4/6 inhibitors as first-line therapy for mBC have poorer treatment outcomes than patients with wild-type BRCA [4, 35]. Accordingly, treatment with olaparib earlier in the disease course may be particularly important in patients with gBRCAm, HER2-negative mBC. The OS, TFST, TSST, and PFS2 findings suggest that olaparib treatment may delay subsequent treatment and progression milestones.
Safety findings were consistent with the well-tolerated and manageable safety profile of olaparib seen in the interim analysis of the LUCY trial and in previous olaparib studies, both in BC and in other tumor types [14, 15, 24, 27]. Although most patients (71.4%) had a dose modification for several reasons, including AEs, the rate of discontinuation from olaparib due to TEAEs was low (6.3%) and similar to that observed in OlympiAD (4.9%) [14]. This suggests that TEAEs were generally effectively managed, allowing sustained treatment with olaparib for as long as patients received a clinical benefit. One patient experienced grade 4 MDS, considered by the investigator to be treatment-related, which led to discontinuation of olaparib treatment. Of note, this patient had previously received both anthracycline- and platinum-based chemotherapy, which are known DNA-damaging agents [36]. It is the only case of MDS that has been reported in the OlympiAD and LUCY trials. To date, no cases of AML have been reported in either trial [14, 15, 27]. In the OlympiA trial in early BC, MDS/AML were reported in two patients in the olaparib arm and three patients in the placebo arm [24]. MDS/AML have also been reported in patients with ovarian and prostate cancer treated with olaparib. These patients had received chemotherapy before exposure to olaparib, which may have included treatments linked to an increased risk of MDS/AML (such as radiotherapy and platinum-based chemotherapy) [36,37,38].
Differences between LUCY and OlympiAD must be considered when comparing findings from the two trials [27]. The LUCY trial was designed to have less stringent eligibility criteria compared with the OlympiAD trial. For example, in LUCY there were no eligibility criteria related to ECOG-PS, whereas all patients enrolled in OlympiAD had to have an ECOG-PS of 0–1. Interestingly, despite this, most patients (n = 250 [98.0%]) enrolled in LUCY had an ECOG-PS of 0–1, perhaps reflecting where physicians see the value of olaparib for patients. Patients enrolled in LUCY were required to have previously received either taxane- or anthracycline-based chemotherapy, whereas patients in OlympiAD must have previously received both taxane- and anthracycline-based chemotherapy. However, 75.3% of patients in LUCY had received both drugs, reflective of standard clinical practice. Therefore, despite the differences in eligibility criteria, the OlympiAD and LUCY trials enrolled similar patient populations that are highly relevant to those encountered in clinical practice. Efficacy measurements differed in the LUCY and OlympiAD studies. Specifically, tumor responses were assessed by the study investigators in the LUCY trial, whereas a more standardized approach was adopted in OlympiAD, where tumor responses were assessed by blinded independent central review [15, 27]. In LUCY, the frequency of patient follow-up for tumor evaluation following a first progression event was not mandated and was instead carried out per local practice and standard of care. This limitation should be taken into consideration when reviewing the intermediate efficacy endpoints within the study.
Other limitations include the limited diversity of the patient population enrolled, suggesting that increased efforts are required to broaden inclusion in clinical trials overall and to better represent the populations at risk. The short period between the protocol amendment permitting the inclusion of patients with an sBRCA mutation (April 27, 2018) and the date of the last patient enrollment (March 21, 2019), as well as the lack of routine screening for sBRCA mutations at the time of the study likely contributed to the low number of patients enrolled into the sBRCAm cohort [35]. As well, the small sample size and missing data limited assessment of the clinical effectiveness of olaparib in patients with an sBRCA mutation. Although no firm conclusions can be drawn from the sBRCAm cohort in this study, a phase II single-arm, proof-of-principle trial has reported encouraging data in mBC [28]. Furthermore, evidence in ovarian and prostate cancer is also supportive of a benefit of PARP inhibition for patients with sBRCA mutations [28,29,30,31,32]. Additional investigations in patients with sBRCA mutations are warranted.
Conclusions
The findings from the final prespecified analysis of the phase IIIb LUCY trial support the clinical effectiveness and safety of olaparib in patients with gBRCAm, HER2-negative mBC in a real-world setting, and may help to guide clinical practice.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
References
Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, Gonzalez-Neira A, Luccarini C et al (2021) Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med 384(5):428–439. https://doi.org/10.1056/NEJMoa1913948
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ et al (2017) Risks of Breast, Ovarian, and contralateral Breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416. https://doi.org/10.1001/jama.2017.7112
Aleskandarany M, Caracappa D, Nolan CC, Macmillan RD, Ellis IO, Rakha EA et al (2015) DNA damage response markers are differentially expressed in BRCA-mutated Breast cancers. Breast Cancer Res Treat 150(1):81–90. https://doi.org/10.1007/s10549-015-3306-6
Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC et al (2021) A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic Breast cancer by germline BRCA mutation status. Oncol Ther 9(2):575–589. https://doi.org/10.1007/s40487-021-00162-4
O’Shaughnessy J, Brezden-Masley C, Cazzaniga M, Dalvi T, Walker G, Bennett J et al (2020) Prevalence of germline BRCA mutations in HER2-negative metastatic Breast cancer: global results from the real-world, observational BREAKOUT study. Breast Cancer Res 22(1):114. https://doi.org/10.1186/s13058-020-01349-9
Pogoda K, Niwińska A, Sarnowska E, Nowakowska D, Jagiełło-Gruszfeld A, Siedlecki J et al (2020) Effects of BRCA germline mutations on triple-negative Breast cancer prognosis. J Oncol 2020:8545643. https://doi.org/10.1155/2020/8545643
O’Connor MJ (2015) Targeting the DNA damage response in cancer. Mol Cell 60(4):547–560. https://doi.org/10.1016/j.molcel.2015.10.040
Tung NM, Garber JE (2018) BRCA1/2 testing: therapeutic implications for Breast cancer management. Br J Cancer 119(2):141–152. https://doi.org/10.1038/s41416-018-0127-5
Hahnen E, Lederer B, Hauke J, Loibl S, Kröber S, Schneeweiss A et al (2017) Germline mutation status, pathological complete response, and disease-free survival in triple-negative Breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol 3(10):1378–1385. https://doi.org/10.1001/jamaoncol.2017.1007
Pohl-Rescigno E, Hauke J, Loibl S, Mobus V, Denkert C, Fasching PA et al (2020) Association of germline variant status with therapy response in high-risk early-stage Breast cancer: a secondary analysis of the GeparOcto randomized clinical trial. JAMA Oncol 6(5):744–748. https://doi.org/10.1001/jamaoncol.2020.0007
Fasching PA, Loibl S, Hu C, Hart SN, Shimelis H, Moore R et al (2018) BRCA1/2 mutations and bevacizumab in the neoadjuvant treatment of Breast cancer: response and prognosis results in patients with triple-negative Breast cancer from the GeparQuinto study. J Clin Oncol 36(22):2281–2287. https://doi.org/10.1200/JCO.2017.77.2285
De Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A et al (2020) Clinical outcome of Breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep 10(1):7073. https://doi.org/10.1038/s41598-020-63759-1
Robson M, Ruddy KJ, Im SA, Senkus E, Xu B, Domchek SM et al (2019) Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic Breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer 120:20–30. https://doi.org/10.1016/j.ejca.2019.06.023
Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B et al (2019) OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic Breast cancer. Ann Oncol 30(4):558–566. https://doi.org/10.1093/annonc/mdz012
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N et al (2017) Olaparib for metastatic Breast cancer in patients with a germline BRCA mutation. N Engl J Med 377(6):523–533. https://doi.org/10.1056/NEJMoa1706450
Hurvitz SA, Goncalves A, Rugo HS, Lee KH, Fehrenbacher L, Mina LA et al (2020) Talazoparib in patients with a germline BRCA-mutated advanced Breast cancer: detailed safety analyses from the Phase III EMBRACA trial. Oncologist 25(3):e439–e50. https://doi.org/10.1634/theoncologist.2019-0493
Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Gonçalves A et al (2020) Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced Breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 31(11):1526–1535. https://doi.org/10.1016/j.annonc.2020.08.2098
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH et al (2018) Talazoparib in patients with advanced Breast cancer and a germline BRCA mutation. N Engl J Med 379(8):753–763. https://doi.org/10.1056/NEJMoa1802905
Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L et al (2020) Outcomes in clinically relevant patient subgroups from the EMBRACA study: talazoparib vs physician’s choice standard-of-care chemotherapy. JNCI Cancer Spectr 4(1):pkz085. https://doi.org/10.1093/jncics/pkz085
European Medicines Agency (2022) Summary of product characteristics (olaparib). https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf. Accessed 15 June 2022
US Food and Drug Administration (2022) Prescribing information. Lynparza (olaparib) tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208558s023lbl.pdf. Accessed 15 June 2022
European Medicines Agency (2022) Summary of product characteristics (talazoparib). https://www.ema.europa.eu/en/documents/product-information/talzenna-epar-product-information_en.pdf. Accessed 06 Sep 2022
US Food and Drug Administration (2018) Prescribing information. Talazenna (talazoparib) capsules, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211651s000lbl.pdf. Accessed 06 Sep 2022
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P et al (2021) Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated Breast cancer. N Engl J Med 384(25):2394–2405
Tutt ANJ, Garber J, Gelber RD, Phillips K-A, Eisen A, Johannsson OT et al (2022) Pre-specified event driven analysis of overall survival (OS) in the olympia phase III trial of adjuvant olaparib (OL) in germline BRCA1/2 mutation (gBRCAm) associated Breast cancer. Ann Oncol 33(5):566–568
European Medicines Agency (2022) CHMP post-authorisation summary of positive opinion for lynparza (olaparib). https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-lynparza-ii-51-g_en.pdf. Accessed 05 Sep 2022
Gelmon KA, Fasching PA, Couch FJ, Balmana J, Delaloge S, Labidi-Galy I et al (2021) Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic Breast cancer in a real-world setting: phase IIIb LUCY interim analysis. Eur J Cancer 152:68–77. https://doi.org/10.1016/j.ejca.2021.03.029
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK et al (2020) TBCRC 048: phase II study of olaparib for metastatic Breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38(36):4274–4282. https://doi.org/10.1200/jco.20.02151
Carreira S, Porta N, Arce-Gallego S, Seed G, Llop-Guevara A, Bianchini D et al (2021) Biomarkers associating with PARP inhibitor benefit in Prostate cancer in the TOPARP-B trial. Cancer Discov 11(11):2812–2827. https://doi.org/10.1158/2159-8290.Cd-21-0007
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R et al (2015) DNA-repair defects and olaparib in metastatic Prostate cancer. N Engl J Med 373(18):1697–1708. https://doi.org/10.1056/NEJMoa1506859
Lee CK, Friedlander ML, Tjokrowidjaja A, Ledermann JA, Coleman RL, Mirza MR et al (2021) Molecular and clinical predictors of improvement in progression-free survival with maintenance PARP inhibitor therapy in women with platinum-sensitive, recurrent Ovarian cancer: a meta-analysis. Cancer 127(14):2432–2441. https://doi.org/10.1002/cncr.33517
Reiss KA, Mick R, O’Hara MH, Teitelbaum U, Karasic TB, Schneider C et al (2021) Phase II study of maintenance rucaparib in patients with platinum-sensitive Advanced Pancreatic Cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol 39(22):2497–2505. https://doi.org/10.1200/JCO.21.00003
Collett D (1994) Modelling survival data in medical research, 1st edn. Springer, New York
Mavrommati I, Johnson F, Echeverria GV, Natrajan R (2021) Subclonal heterogeneity and evolution in Breast cancer. NPJ Breast Cancer 7(1):155. https://doi.org/10.1038/s41523-021-00363-0
Collet L, Péron J, Penault-Llorca F, Pujol P, Lopez J, Freyer G et al (2022) PARP inhibitors: a major therapeutic option in endocrine-receptor positive Breast cancers. Cancers 14(3):599. https://doi.org/10.3390/cancers14030599
Cheung-Ong K, Giaever G, Nislow C (2013) DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol 20(5):648–659. https://doi.org/10.1016/j.chembiol.2013.04.007
Zhu J, Tucker M, Wang E, Grossman JS, Armstrong AJ, George DJ et al (2017) Acute Myeloid Leukemia after olaparib treatment in metastatic castration-resistant Prostate cancer. Clin Genitourin Cancer 15(6):e1137–e1141
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J et al (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33(3):244–250
Acknowledgements
The authors thank the patients and their families for their participation in this study, the on-site personnel, and members of the independent data and safety monitoring committee. Medical writing support was provided by Elizabeth Gandhi, PhD, and Michelle Antoni, PhD, of Oxford PharmaGenesis Ltd, UK, and Sara Shaw, PhD CMPP, of BOLDSCIENCE Ltd, funded by AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funding source was involved in the study design, analysis, data interpretation, writing of the manuscript, and the decision to submit the article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
KAG and SD contributed to the conception/design of the study. JB, PAF, FJC, SD, ILG, JO, YHP, AFE, BY, HB, AG, ZK, AS, TJ, JHS, EP, GM, SA, CT, TWPS, AAT, and KAG were involved in the collection of data and/or assembly of data. KAG, JB, PAF, FJC, EJ, KB, and IG conducted data analysis and interpretation. All authors contributed to writing the original draft and reviewing and editing the same, and provided final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The following authors have received compensation for serving as a consultant, invited speaker, or medical writer, or they or the institutions they work for have received research support from the companies or organizations indicated: J Balmaña (AstraZeneca, PharmaMar, and Pfizer); PA Fasching (Agendia, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Hexal, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, and Seagen); FJ Couch (Ambry Genetics, AstraZeneca, GRAIL, Qiagen, and US Oncology); S Delaloge (AstraZeneca, Besins Healthcare, Bristol Myers Squibb, Cellectis, Eli Lilly, Exact Sciences, GE, Novartis, Orion, Pfizer, Pierre Fabre, Puma Biotechnology, Rappta Therapeutics, Roche Genentech, Sanofi, Seagen, Servier, and Taiho Pharma); I Labidi-Galy (AstraZeneca and PharmaMar); J O’Shaughnessy (AbbVie, Agendia, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Genentech, Genomic Health, GRAIL, HERON, Immunomedics, Ipsen, Jounce Therapeutics, Lilly, Merck Sharp & Dohme, Myriad Pharmaceuticals, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Samsung, Sanofi, Seattle Genetics, and Syndax); YH Park (AstraZeneca, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, and Roche); B You (AstraZeneca); H Bourgeois (Daiichi Sankyo, Lilly, and Novartis); A Goncalves (AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Roche, Sanofi, and Seagen); Z Kemp (AstraZeneca and Lilly); JH Sohn (AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Sanofi); S Aksoy (AstraZeneca, Bristol Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and Roche); CV Timcheva (AstraZeneca, Eli Lilly, i3 Research, Merck Sharp & Dohme, Novartis, Parexel, and Roche); T-W Park-Simon (AstraZeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Seagen); A Antón-Torres (AstraZeneca, Eli Lilly, Daichi Sankyo, Gilead, Roche, and Seagen); KA Gelmon (AstraZeneca, Ayala, Bristol Myers Squibb, Eli Lilly, Gilead Sciences, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Seagen). E John, K Baria, and I Gibson are employees and/or stockholders of AstraZeneca. T Jankowski, G Mukhametshina, E Poddubskaya, AF Eisen, and A Swampillai have declared no conflicts of interest.
Ethical approval
The trial was performed in accordance with the Declaration of Helsinki, International Conference on Harmonisation of Good Clinical Practice guidelines, applicable regulatory requirements, and the AstraZeneca Global Standard on Bioethics [33]. The trial protocol was approved by independent ethics review committees at all participating institutions/countries.
Consent to participate
All patients provided written informed consent to participate.
Consent to publish
All patients provided written informed consent to publish.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The LUCY investigators: Participating countries and collaborating investigators are listed in Supplementary File
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balmaña, J., Fasching, P.A., Couch, F.J. et al. Clinical effectiveness and safety of olaparib in BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: final analysis of LUCY. Breast Cancer Res Treat 204, 237–248 (2024). https://doi.org/10.1007/s10549-023-07165-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07165-x