Abstract
Background
The efficacy and safety of olaparib compared with placebo in the subset of patients from Japan in the phase 3 OlympiA trial (NCT02032823) are reported here and contextualized with reference to the global OlympiA population.
Methods
Patients with germline BRCA1 and/or BRCA2 pathogenic variants and HER2-negative, high-risk early breast cancer who had received neoadjuvant or adjuvant chemotherapy and completed local treatment were eligible. Patients were randomized 1:1 to receive olaparib or placebo for 1 year. Primary endpoint: invasive disease-free survival (IDFS). Secondary endpoints: distant disease-free survival (DDFS), overall survival (OS), and safety. Data are reported from the first pre-specified interim analysis (data cut-off [DCO] March 27, 2020) and the second, event driven, pre-specified interim analysis of OS (DCO July 12, 2021) in patients from Japan.
Results
140 patients were randomized in Japan (olaparib, n = 64; placebo, n = 76). At the first pre-specified interim analysis (median follow-up: 2.9 years), hazard ratios (HRs) for adjuvant olaparib compared with placebo were 0.5 for IDFS (95% confidence interval [CI] 0.18–1.24) and 0.41 for DDFS (95% CI 0.11–1.16). At the second pre-specified interim analysis of OS, three deaths occurred in the olaparib group versus six deaths in the placebo group (HR, 0.62 [95% CI 0.13–2.36]). Findings were consistent with those for the global population. No new safety signals were observed.
Conclusions
While this analysis in a Japanese subset of patients was not powered to detect population-related treatment differences, efficacy and safety analysis results were consistent with the global OlympiA population, suggesting the findings from the global study are generalizable to clinical practice in Japan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olaparib is an inhibitor of the poly (adenosine diphosphate–ribose) polymerase (PARP) family of enzymes, which exploits the principle of synthetic lethality to selectively kill tumor cells deficient in homologous recombination repair pathways, such as those harboring loss-of-function mutations in BRCA1 and/or BRCA2 genes [1, 2]. Olaparib has been extensively studied and is widely approved for use in cancers where homologous recombination repair deficiencies are common, including metastatic breast cancer (mBC) or early breast cancer (eBC) [3, 4].
The multinational phase 3 OlympiA trial compared 1 year of adjuvant olaparib with placebo in patients with germline BRCA1 and/or BRCA2 pathogenic variants (gBRCA1/2pv) and human epidermal growth factor receptor 2 (HER2)-negative, high-risk eBC. The first pre-specified interim analysis (data cut-off [DCO] March 27, 2020) demonstrated that olaparib clinically and significantly prolonged invasive disease-free survival (IDFS) and distant disease-free survival (DDFS) compared with placebo with no new safety signals [4]. While olaparib was associated with fewer deaths than placebo at the time of the first interim analysis, the between-group difference did not meet the pre-specified boundary for statistically significant differences in overall survival (OS) (p < 0.01) [4]. However, in a subsequent event-driven second interim analysis when 330 IDFS events had been reported (DCO July 12, 2021), adjuvant olaparib was associated with a statistically significant and clinically meaningful OS improvement compared with placebo (hazard ratio, 0.68; 98.5% CI 0.47–0.97; p = 0.009) [5]. Updated analyses of event-free rates at this second pre-specified interim analysis continued to show improvements for olaparib versus placebo; 4-year IDFS was 83% for olaparib versus 75% for placebo, and 4-year DDFS was 87 versus 79%, respectively [5].
Results from the OlympiA study led to the approval of olaparib for the adjuvant treatment of adult patients with germline BRCA-mutated, HER2-negative, high-risk eBC who received prior neoadjuvant or adjuvant chemotherapy ([N]ACT) in multiple countries.
Here, we report the efficacy and safety of olaparib compared with placebo in the subset of patients from Japan and contextualize the findings with reference to the outcomes in the global OlympiA population.
Methods
Study design
Key aspects of the methodology used in the OlympiA clinical trial (NCT02032823) will be summarized here, having been described in detail previously [4].
OlympiA is an ongoing, prospective, multicenter, multinational, double-blind, randomized, phase 3 clinical trial with an expected overall follow-up of 10 years. Enrolled patients had gBRCA1/2pv determined by local or central testing, with HER2-negative, hormone receptor-positive or negative, high-risk eBC. Patients were required to have completed definitive local treatment, including radiotherapy if indicated, at least 2 weeks before trial entry and to have received at least six cycles of (N)ACT containing anthracyclines, taxanes, or a combination of both. Previous platinum-based chemotherapy (CT) treatment for prior cancer, including eBC, was also permitted but was not a requirement for eligibility. Adjuvant endocrine therapy for patients with hormone receptor-positive eBC was to be administered according to local guidelines during the study treatment. Adjuvant bisphosphonates and denosumab were allowed concurrently with olaparib treatment according to investigator practice. ACT after surgery was not allowed in patients who received NACT [4].
Patients with hormone receptor-positive eBC were considered high risk if they had at least four pathologically confirmed positive lymph nodes at initial surgery prior to ACT or had evidence of lack of pathological complete response (non-pCR) to NACT with a score of at least 3 for the CPS + EG staging system [4], which estimates the probability of relapse following NACT based on baseline clinical and post-NACT pathological stages, estrogen receptor status, and nuclear grade (scores range from 0 to 6, with higher scores indicating worse prognosis) [6]. Patients with early triple-negative breast cancer (TNBC) were considered high risk if they were axillary node-positive or axillary node-negative with an invasive primary tumor measuring at least 2 cm at initial surgery prior to ACT or had a non-pCR following NACT [4].
Treatment and assessments
Patients were randomized in a 1:1 ratio to receive olaparib 300 mg or matching placebo tablets, taken orally twice daily for 1 year. Patients were stratified according to hormone receptor status (positive or negative), timing of prior CT (NACT or ACT), and prior use of platinum-based CT (yes or no) [4].
Following randomization, patients were assessed for disease recurrence through physical examinations and medical history every 4 weeks for 24 weeks, then every 3 months through year 2, every 6 months in years 3–5, and annually after that. Breast imaging was performed every 12 months, with other imaging investigations performed at the investigator’s discretion when symptoms, physical examination findings, or laboratory results suggested the possibility of disease recurrence [4].
Outcomes
The primary endpoint of the OlympiA trial was IDFS, defined as the time from randomization until the date of the first occurrence of one of the following events: ipsilateral invasive breast tumor, locoregional invasive disease, distant recurrence, contralateral invasive breast cancer (BC), second primary invasive cancer, or death from any cause [4, 7]. Secondary endpoints included DDFS (defined as the time from randomization until documented evidence of the first distant recurrence of breast cancer or death) and OS (defined as the time from randomization until death from any cause [4, 7]). Safety outcomes were also investigated as a secondary endpoint, assessed using the Common Terminology Criteria for Adverse Events (CTCAE), with adverse events (AEs) of special interest comprising pneumonitis, radiation pneumonitis, myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and new primary cancer other than MDS or AML [4].
An independent external data and safety monitoring committee reviewed the results of the planned first interim analysis of the primary endpoint (IDFS). They recommended proceeding with the analysis and reporting [4].
Statistical analyses
Efficacy analyses were based on the intention-to-treat population, including all patients who underwent randomization. Survival functions were estimated utilizing Kaplan–Meier curves. The stratified Cox proportional hazards model was used to estimate hazard ratio (HRs) and confidence intervals (CIs), and a comparison of survival between treatment groups was performed using the stratified log-rank test. Safety was assessed in the population of patients who received at least one dose of olaparib or placebo [4].
Analysis of the subset of patients from Japan was performed using the global OlympiA first pre-specified interim analysis DCO date of March 27, 2020. Results for primary and key secondary endpoints of IDFS and DDFS, respectively, are presented for the subset of patients from Japan using this data. Following the event-driven pre-specified second interim analysis of OS for the global OlympiA population at DCO on July 12, 2021, the results of OS for the subset of patients from Japan are also presented here, as well as 4-year data for the primary and key secondary endpoints. Safety data are presented using data from the pre-specified second interim analysis. All data from the subset of patients from Japan is descriptive. The study was not powered to detect treatment differences in the subset of patients from Japan at either DCO.
Results
Patient disposition
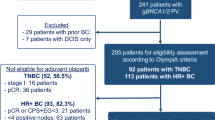
In total, 1836 patients were randomized from June 2014 through to May 2019 to the global OlympiA trial [4]. Of these patients, 140 were randomized in Japan, with 64 to receive olaparib and 76 to receive placebo, all of whom received the assigned study treatment. During screening for the global OlympiA population, 10,514 patients underwent prospective gBRCA central testing, of whom 1383 (13.2%) had a confirmed gBRCA1/2pv. Of the total population that underwent central gBRCA testing, 1344 were from Japan, of whom 232 (17.3%) had a confirmed gBRCA1/2pv. At the time of the first pre-specified interim analysis, median follow-up was 2.9 years in the subset of patients from Japan and 2.5 years in the global OlympiA intention-to-treat population [4].
Baseline demographics and disease characteristics
All patients in the subset from Japan were female, with a median age of 43 years (Table 1); 15.7% had hormone receptor-positive BC, and 84.3% had TNBC. Among the subset from Japan, gBRCA1pv were present in 73.4% of patients in the olaparib group and 63.2% in the placebo group, while gBRCA2pv were present in 26.6% and 36.8% of patients, respectively. No patients had both a gBRCA1pv and gBRCA2pv. Of patients that harbored a gBRCA1pv (n = 95), 7 (7.4%) had hormone receptor-positive BC and 88 (92.6%) had TNBC, while in patients with a gBRCA2pv (n = 45), 15 (33.3%) had hormone receptor-positive BC and 30 (66.7%) had TNBC. Across both treatment groups, 50.0% had received prior ACT, and 50.0% had prior NACT. Demographics and characteristics were generally similar between the subset of patients from Japan and the global OlympiA population, with the notable exception that substantially fewer patients in the subset from Japan (3.6%) had received prior platinum-based CT compared with the global OlympiA population (26.5%) (Table 1).
Clinical efficacy
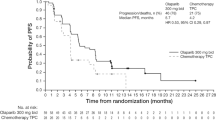
At the time of the first pre-specified interim analysis, the HR for IDFS in the olaparib group (6 events) compared with the placebo group (15 events) was 0.50 (95% CI 0.18–1.24) in the subset of patients from Japan (Fig. 1a). These findings were consistent with those in the global OlympiA population, for which a 42% reduction in the risk of invasive disease recurrence or death was observed for olaparib (106 IDFS events) compared with placebo (178 IDFS events) (HR, 0.58 [95% CI 0.46–0.74; p < 0.0001]) (Fig. 1b). In the subset of patients from Japan, 87.1% (95% CI 72.5–94.2%) of patients in the olaparib group were alive and free of invasive disease at 3 years compared with 82.4% (95% CI 70.9–89.7%) of patients in the placebo group; this was consistent with the global OlympiA population, in which 85.9% (95% CI 82.8–88.4%) and 77.1% (95% CI 73.7–80.1%) of the olaparib group and the placebo group, respectively, were alive and free of invasive disease at 3 years. Analysis of IDFS at the time of the pre-specified second interim analysis for the subset of patients from Japan showed similar results to the first pre-specified interim analysis (Supplemental Table 1). At this time, 4-year IDFS was 86.2% (95% CI 74.1–92.9%) for patients in the olaparib group compared to 78.9% (95% CI 67.2–86.8%) for patients in the placebo group; consistent with the global OlympiA population [5].
Invasive disease-free survival in the subset of patients from a Japan and b the global OlympiA population. IDFS at first pre-specified interim analysis (data cut-off March 27, 2020) in the subset of patients from Japan (panel a) and in the global OlympiA population (panel b). IDFS was defined as the time from randomization until the date of one of the following events: ipsilateral invasive breast tumor, locoregional invasive disease, distant recurrence, contralateral invasive breast cancer, second primary invasive cancer, or death from any cause. Data for patients without a documented event of invasive disease or death were censored at the date they were last known to be disease-free. Panel b From New England Journal of Medicine. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Volume 384., Page No 2399. Copyright © (2021) Massachusetts Medical Society. Reprinted with permission. CI confidence interval, HR hazard ratio, IDFS invasive disease-free survival, No., number
At the time of the first pre-specified interim analysis, the HR for DDFS in the olaparib group (4 DDFS events) compared with the placebo group (13 DDFS events) was 0.41 (95% CI 0.11–1.16) in the subset of patients from Japan (Fig. 2a). This was consistent with the global OlympiA population, in which the risk of distant disease or death was reduced by 43% in the olaparib group (89 events) compared with the placebo group (152 events) (HR, 0.57 [95% CI 0.44–0.74; p < 0.0001]) (Fig. 2b). The observed 3-year DDFS rate in the subset of patients from Japan was 91.7% (95% CI 78.7–96.9%) in the olaparib group compared with 87.1% (95% CI 76.5–93.1%) in the placebo group, consistent with the global OlympiA population in which the 3-year DDFS rate was 87.5% (95% CI 84.6–89.9%) in the olaparib group and 80.4% (95% CI 77.2–83.3%) in the placebo group. Analysis of DDFS at the time of the pre-specified second interim analysis showed similar results (Supplemental Table 1). At the time of the second interim analysis, 4-year DDFS was 87.8% (95% CI 76.0–94.0%) in the olaparib group compared to 81.9% (95% CI 69.9–89.5%) in the placebo group in the subset of patients from Japan; consistent with the global OlympiA population [5].
Distant disease-free survival in the subset of patients from a Japan and b the global OlympiA population. DDFS at first pre-specified interim analysis (data cut-off March 27, 2020) in the subset of patients from Japan (panel a) and in the global OlympiA population (panel b). DDFS was defined as the time from randomization until documented evidence of the first distant recurrence of breast cancer or death. Distant recurrence includes the following events: metastatic breast cancer; death attributable to any cause, including breast cancer, non-breast cancer, or unknown cause; and second primary non-breast invasive cancer. Evidence of distant recurrence requires either radiologic examination or histopathological confirmation by biopsy. Panel b From New England Journal of Medicine. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Volume 384., Page No 2399. Copyright © (2021) Massachusetts Medical Society. Reprinted with permission. CI confidence interval, DDFS distant disease-free survival, HR hazard ratio, No. number
At the time of the first pre-specified interim analysis, seven deaths (5.0%) had been reported in patients from Japan, three in the olaparib group and four in the placebo group (HR, 0.94 [95% CI 0.19–4.28]). At the subsequent pre-specified second interim analysis, the median OS follow-up time in the subgroup of patients from Japan was 3.9 and 4.2 years for the olaparib group and placebo group, respectively. At this time, nine deaths (6.4%) had been reported, three in the olaparib group and six in the placebo group (HR, 0.62 [95% CI 0.13–2.36]). At 4 years from randomization, the percentage of patients alive was 94.6% (95% CI 84.0–98.3%) in the olaparib group and 91.8% (95% CI 82.5–96.2%) in the placebo group. These findings are consistent with results from the global OlympiA population, where olaparib significantly improved OS compared to placebo in the second interim analysis; HR, 0.68 (98.5% CI 0.47–0.97; p = 0.009) [5].
Safety
All 140 patients from Japan were included in the safety analysis (DCO July 12, 2021). The median actual treatment exposure was 353.0 days in the olaparib group and 364.0 days in the placebo group, while the median relative dose intensity was 98.9 and 100%, respectively. The median percentage of the intended dose received was 95.9% for olaparib and 99.0% for placebo.
The overall safety reported in the subset of patients from Japan was consistent with the global OlympiA population (Table 2). Most AEs were mild or moderate in severity (Grade ≤ 2). The most frequently reported Grade 3 or higher AEs in the olaparib group of the subset of patients from Japan were anemia (14.1%), neutrophil count decreased (10.9%), and white blood cell count decreased (7.8%), comparable to the global OlympiA population, in which the most frequently reported Grade 3 or higher in the olaparib group were anemia (8.7%), neutrophil count decreased (4.9%), and white blood cell count decreased (3.0%) (Table 3).
Serious AEs were reported at a similar rate between the olaparib group (6.3%) and the placebo group (5.3%) in the subset from Japan, rates comparable to the global OlympiA population (Table 2).
Among the subset of patients from Japan, none in the olaparib group and one patient in the placebo group (1.3%) had developed AML; in the global OlympiA population, two (0.2%) and three (0.3%) patients had developed AML in the olaparib group and placebo group, respectively. None of the patients from Japan in the olaparib group received a blood transfusion. In contrast, one patient (0.7%) in the placebo group received multiple blood products as part of management for AML.
Among the patients from Japan, four (6.3%; breast cancer [n = 2], colorectal cancer, and gastric cancer) patients in the olaparib group and three (3.9%; malignant lung neoplasm, fallopian tube cancer and transitional cell carcinoma) patients in the placebo group had developed new primary cancers > 30 days after finishing treatment. One patient in the placebo group also developed ovarian cancer with onset within 30 days of stopping study treatment. In the global OlympiA population, 21 (2.3%) and 36 (4.0%) patients in the olaparib- and placebo groups, respectively, had developed new primary cancers [5]. Radiation pneumonitis occurred in one patient (1.6%) from Japan in the olaparib group and two patients (2.6) from Japan in the placebo group within 30 days of stopping study treatment.
Among the subset of patients from Japan, seven patients (10.9%) in the olaparib group and one patient (1.3%) in the placebo group permanently discontinued study treatment owing to AEs, which was consistent with the observation of a higher number of permanent discontinuations due to AEs in the global OlympiA population in the olaparib arm (98 [10.8%] in the olaparib group and 42 [4.6%] in the placebo group). All deaths in patients from Japan were the results of breast cancer, except for one patient in the placebo group who died due to AML (considered an AE).
Discussion
The present analysis was conducted to support the approval of olaparib as adjuvant therapy in BRCA-mutated, HER2-negative eBC at high risk of recurrence by the Japanese Pharmaceutical and Medical Devices Agency. The results of the descriptive analyses for IDFS and DDFS in the subgroup of patients from Japan were consistent with the definitive results reported in the overall study population, which demonstrated statistically significant and clinically meaningful improvements in the primary endpoint of IDFS and the secondary endpoint of DDFS for the global OlympiA population at the time of the first pre-specified interim analysis. OS results in the subset of patients from Japan at the time of the pre-specified second interim analysis were also consistent with results reported in the global OlympiA population, where statistical significance was demonstrated [5]. Consistency between data from the subset of patients from Japan and the global OlympiA population was also seen for 4-year event-free rates at this second analysis point [5].
Safety data for the subset of patients from Japan were consistent with the known safety profile of olaparib in eBC and in the treatment of other advanced or metastatic cancers [8], and no new safety signals were observed. Most AEs were mild or moderate in severity. While there was a slightly higher incidence of Grade ≥3 AEs in patients receiving olaparib in the subset from Japan than in the global OlympiA population, the number of patients requiring permanent discontinuation of treatment due to AEs was similar between populations. Hematological toxicities occurred at a slightly higher rate in the subset of patients from Japan and are also more common in Asian patients than non-Asian patients following the administration of CT and targeted therapies for breast cancer treatment [9]. Hematological AEs can generally be managed using an effective dose adjustment strategy [9], which could further reduce the already low number of patients requiring permanent discontinuation of study treatment due to AEs. AML and MDS were considered AEs of special interest in the OlympiA trial. As such, it is reassuring that no cases were reported in the olaparib group for the subset of patients from Japan, though long-term follow-up is still warranted for a complete assessment of risk.
The OlympiA study was designed to assess the efficacy and safety of olaparib in patients with gBRCA1/2pv and high-risk, HER2-negative eBC, irrespective of hormone receptor status. While olaparib is the only treatment option specifically for patients with gBRCA1/2pv, other treatment options are available in Japan for use as adjuvant treatment in patients at high risk of recurrence. As such, physicians will need to choose between olaparib and other agents available in this patient population.
For patients with TNBC at high risk of recurrence, pembrolizumab is the only approved treatment option. Pembrolizumab can be used in combination with NACT and continued as a single-agent adjuvant treatment after surgery based on the results of the KEYNOTE-522 trial, which demonstrated a significant difference in pCR rate with the addition of pembrolizumab to NACT (64.8% [95% CI 59.9–69.5%]) compared with CT alone (51.2% [95% CI 44.1–58.3%]), and significant event-free survival (HR, 0.63 [95% CI 0.48–0.82]) [10, 11]. Assessment of the Asian subset of patients in the KEYNOTE-522 study, which included patients from Japan, Korea, Taiwan, and Singapore, demonstrated consistent findings with the overall study population [12]. However, the KEYNOTE-522 trial did not evaluate the gBRCA1/2pv status of participating patients; outcomes in this specific population of patients are unknown.
Reflecting the lack of clarity regarding clinical benefit of adjuvant capecitabine, treatment is not approved or reimbursed in Japan, but is recommended by the Japanese Breast Cancer Society clinical practice guidelines and Pan-Asian guidelines as a treatment option for patients with TNBC not achieving pCR following NACT [13, 14]. Data from the CREATE-X trial in patients from Japan and South Korea showed that adjuvant capecitabine demonstrated significantly longer disease-free survival (HR, 0.58 [95% CI 0.39–0.87]) and OS (HR, 0.52 [95% CI 0.30–0.90]) in patients with TNBC following standard NACT [15]. Although capecitabine has not been specifically evaluated in patients with gBRCA1/2pv high-risk eBC, adjuvant capecitabine has been studied in patients with basal subtype TNBC, the subtype patients with gBRCA1/2pv typically develop [16, 17]. In a preplanned subgroup analysis of the GEICAM/CIBOMA study, which investigated adjuvant capecitabine following standard (N)ACT in TNBC, benefit of capecitabine was observed in non-basal patients compared to patients with the basal phenotype [16]. Patients with basal subtype TNBC also have been shown to have a worse prognosis regardless of treatment with either adjuvant capecitabine or platinum chemotherapy compared to patients with nonbasal subtype in the ECOG-ACRIN EA113127 randomized trial, highlighting the need for alternative treatment approaches for patients with basal subtype TNBC [17]. Preliminary research suggests the addition of capecitabine to conventional ACT may be more effective in non-BRCA1-like TNBC than in BRCA1-like TNBC [18]. Currently, more research is needed to elucidate the benefits of capecitabine in patients with gBRCA1/2pv.
For patients with high-risk, hormone-receptor-positive/HER2-negative eBC, abemaciclib is a treatment option in Japan based on the results from the monarchE trial. Abemaciclib, when combined with endocrine therapy, resulted in a significant difference in IDFS compared with endocrine therapy alone (HR, 0.70 [95% CI 0.59–0.82]), although survival benefits have not yet been reported [19]. Data from patients from Japan treated in the monarchE trial has not yet been reported. However, data analysis in advanced breast cancer showed that abemaciclib, in combination with endocrine therapy in the East Asian populations, provided consistent results with the overall population [20]. It should be noted that, as in the KEYNOTE-522 trial, monarchE was not designed to assess activity in patients with gBRCA1/2pv, and there is evidence to suggest that hormone-receptor-positive mBC and gBRCA2pv may have poor outcomes when treated with cyclin-dependent kinase 4 and 6 inhibitors [21,22,23]. As such, the specific efficacy of abemaciclib in patients with gBRCA1/2pv and high-risk eBC needs to be established to support use.
Potential limitations of the present analysis are the relatively small number of patients in the subset from Japan and the fact that the analysis of this subset of patients was not pre-specified and is descriptive. The lower percentage of patients in the subset from Japan who had received prior platinum-based CT compared with the global OlympiA population should also be considered when interpreting the results. This difference likely resulted from the fact that the use of cisplatin is not covered by insurance for patients with eBC in Japan [14].
To our knowledge, this analysis is the first to assess the clinical benefit and safety of a PARP inhibitor for use as adjuvant therapy in patients with gBRCA1/2pv eBC treated in Japan. The consistency of results between the subset of patients from Japan and the global OlympiA population supports the clinical benefit of adjuvant olaparib for patients with gBRCA1/2pv and HER2-negative, high-risk eBC in Japan after completion of local treatment and neoadjuvant or adjuvant CT. The OlympiA trial is ongoing, with 10-year patient follow-up to provide descriptive efficacy and safety analyses.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with the AstraZeneca data sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Change history
12 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12282-023-01468-z
References
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–405.
Geyer CE Jr, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high risk, early breast cancer. Ann Oncol. 2022;33:1250–68.
Mittendorf EA, Jeruss JS, Tucker SL, Kolli A, Newman LA, Gonzalez-Angulo AM, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29:1956–62.
Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32.
Guo XX, Wu HL, Shi HY, Su L, Zhang X. The efficacy and safety of olaparib in the treatment of cancers: a meta-analysis of randomized controlled trials. Cancer Manag Res. 2018;10:2553–62.
Lu YS, Yeo W, Yap YS, Park YH, Tamura K, Li H, et al. An overview of the treatment efficacy and side effect profile of pharmacological therapies in Asian patients with breast cancer. Target Oncol. 2021;16:701–41.
Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–67.
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21.
Dent R, Cortes J, Pusztai L, McArthur HL, Kuemmel S, Bergh J, et al. 1O KEYNOTE-522 Asian subgroup: phase III study of neoadjuvant pembrolizumab (pembro) vs placebo (pbo) + chemotherapy (chemo) followed by adjuvant pembro vs pbo for early triple-negative breast cancer (TNBC). Ann Oncol. 2020;31:S1241–2.
Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, et al. The Japanese Breast Cancer Society clinical practice guidelines for systemic treatment of breast cancer. Breast Cancer. 2020;27:322–31.
Park YH, Senkus-Konefka E, Im SA, Pentheroudakis G, Saji S, Gupta S, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31:451–69.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59.
Lluch A, Barrios CH, Torrecillas L, Ruiz-Borrego M, Bines J, Segalla J, et al. Phase III trial of adjuvant capecitabine after standard neo-/adjuvant chemotherapy in patients with early triple-negative breast cancer (GEICAM/2003-11_CIBOMA/2004-01). J Clin Oncol. 2020;38:203–13.
Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J Clin Oncol. 2021;39:2539–51.
de Boo LW, Jozwiak K, Joensuu H, Lindman H, Lauttia S, Opdam M, et al. Adjuvant capecitabine-containing chemotherapy benefit and homologous recombination deficiency in early-stage triple-negative breast cancer patients. Br J Cancer. 2022;126:1401–9.
Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–81.
Toi M, Inoue K, Masuda N, Iwata H, Sohn J, Hae Park I, et al. Abemaciclib in combination with endocrine therapy for East Asian patients with HR+, HER2- advanced breast cancer: MONARCH 2 & 3 trials. Cancer Sci. 2021;112:2381–92.
Bruno L, Ostinelli A, Waisberg F, Enrico D, Ponce C, Rivero S, et al. Cyclin-dependent kinase 4/6 inhibitor outcomes in patients with advanced breast cancer carrying germline pathogenic variants in DNA repair-related genes. JCO Precis Oncol. 2022;6: e2100140.
Kim JY, Oh JM, Park YH, Ahn JS, Im YH. Which clinicopathologic parameters suggest primary resistance to palbociclib in combination with letrozole as the first-line treatment for hormone receptor-positive, HER2-negative advanced breast cancer? Front Oncol. 2021;11: 759150.
Safonov A, Bandlamudi C, Tallón de Lara P, Ferrarom E, Derakhshan F, Will M, et al. Comprehensive genomic profiling of patients with breast cancer identifies germline-somatic interactions mediating therapy resistance. Cancer Res. 2022;82:GS4-08.
Acknowledgements
The authors thank the patients and their families, the staff members of the trial partners (Breast International Group, NRG Oncology, Frontier Science, AstraZeneca, Merck Sharp & Dohme, and the National Cancer Institute), the current and former members of the trial committees, and the investigators. Medical writing support, under the direction of the authors in accordance with guidelines for Good Publication Practice, was provided by Lorena Tonarelli, M.Sc., Tamsin Chambers, Ph.D., and Aaron Borg, Ph.D., of PharmaGenesis Cambridge, Cambridge, UK, and was funded by AstraZeneca, as part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Additional medical writing support was also provided by Suzanne Patel, Ph.D., of BOLDSCIENCE.
Funding
This study was supported by grants (U10CA180868, UG1CA189867, and U10CA180822) from the National Cancer Institute and by funding and the provision of olaparib and placebo by AstraZeneca as part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design or data interpretation and drafting of the manuscript. HY, MT, ST, SN, and TT contributed to data collection. All authors critically reviewed the manuscript and approved the final submitted version.
Corresponding author
Ethics declarations
Conflicts of interest
Hideko Yamauchi reports research funding from AstraZeneca and Eiken. Masakazu Toi reports research grants from AFI technology, Astellas, AstraZeneca, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly and companies, GL Science, JBCRG assoc., KBCRN assoc., Luxonus, Nippon Kayaku, Pfizer, Sanwa Shurui, Shimadzu, Shionogi, Taiho, Takeda, and Yakult; lecture chair or honoraria from AstraZeneca, Chugai, Daiichi-Sankyo, Devicore medical Japan, Eisai, Eli Lilly and companies, Exact Science, Kyowa-Kirin, Merck Sharp & Dohme Corp, Nippon Kayaku, Novartis, Pfizer, Shimadzu, Sysmex, Taiho, Takeda, and Yakult; participation on advisory boards with Athenex Oncology, Bertis, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly and companies, Kansai Medical Net, and Terumo; being a member of the board of directors (with no salary) for JBCRG assoc., KBCRN assoc., and NPO org. OOTR; being an associate editor for Asian Journal of Breast Surgery, Asian Journal of Surgery, Breast Cancer Research and Treatment, British Journal of Cancer, Cancer Science, Frontiers in Women’s Cancer, and Scientific Reports; and being a deputy editor for International Journal of Oncology. Shin Takayama reports support for the present manuscript from AstraZeneca. Seigo Nakamura reports support for the present manuscript and honoraria from AstraZeneca. Toshimi Takano reports lecture honoraria from Celltrion, Chugai, Daiichi-Sankyo, Eisai, and Eli Lilly and companies. Karen Cui reports an advisory or officer position with AstraZeneca; and ownership of stock in AstraZeneca, and Bristol-Myers Squibb. Christine Campbell reports salary support from AstraZeneca for work involved in the conduct and management of the trial, including travel to study management meetings, paid to her institution via contract with non-profit organizations; and an advisory or officer position with Frontier Sciences (Scotland). Liesbet De Vos reports receiving funding to her institution from AstraZeneca, Biovica, GlaxoSmithKline, Novartis, Pfizer, Roche/Genentech, Sanofi, and Servier; Royalties or licenses to her institution from Agendia; and an advisory or officer position with Breast International Group. Charles Geyer reports grants to his institution from AbbVie, AstraZeneca, Daiichi-Sankyo, and Genentech/Roche; consulting fees from Athenex; medical writing payment or honoraria from AbbVie, and Genentech/Roche; support for attending meetings and/or travel from AstraZeneca, Daiichi-Sankyo, and Genentech/Roche; compensated participation on advisory boards for Exact Sciences; and uncompensated participation on advisory boards for Daiichi-Sankyo, Genentech/Roche, and SeaGen. Andrew Tutt reports consulting/advisor/honoraria from Pfizer, Artios, Prime Oncology, Gilead, Merck KGaA; Advisory Board funds to his institution from Gilead, AstraZeneca; Research funding to his institution from AstraZeneca, Merck KGaA; expert testimony fees from EM Partners; Stocks–Inbiomotion. Royalty associated payments—ICR rewards to inventor's scheme payments associated with patent for the use of PARP inhibitors in DNA deficient cancers, licensee—AstraZeneca. Other, Grant funded by Breast Cancer Now (BCN) and Cancer Research UK (CRUK) to study homologous recombination deficient breast and other cancers, BCN/CRUK receive payments associated with a patent for the use of PARP inhibitors in DNA deficient cancers, licensee—AstraZeneca.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, as well as Good Clinical Practice guidelines and the AstraZeneca policy on bioethics.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised for retrospective open access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yamauchi, H., Toi, M., Takayama, S. et al. Adjuvant olaparib in the subset of patients from Japan with BRCA1- or BRCA2-mutated high-risk early breast cancer from the phase 3 OlympiA trial. Breast Cancer 30, 596–605 (2023). https://doi.org/10.1007/s12282-023-01451-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01451-8