Abstract
Background
Breast density is strongly related to breast cancer. Identifying associations between environmental exposures and density may elucidate relationships with breast cancer. Metals and polycyclic aromatic hydrocarbons (PAHs) may influence breast density via oxidative stress or endocrine disruption.
Methods
Study participants (n = 222,581) underwent a screening mammogram in 2011 at a radiology facility in the Breast Cancer Surveillance Consortium. Zip code residential levels of airborne PAHs and metals (arsenic, cadmium, chromium, cobalt, lead, manganese, mercury, nickel, and selenium) were assessed using the 2011 EPA National Air Toxics Assessment. Breast density was measured using the Breast Imaging–Reporting and Data System (BI-RADS) lexicon. Logistic regression was used to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CI) for the individual air toxics and dense breasts (BI-RADS 3 or 4). Weighted quantile sum (WQS) regression was used to model the association between the air toxic mixture and density.
Results
Higher residential levels of arsenic, cobalt, lead, manganese, nickel, or PAHs were individually associated with breast density. Comparing the highest to the lowest quartile, higher odds of having dense breasts were observed for cobalt (OR = 1.60, 95% CI 1.56–1.64) and lead (OR = 1.56, 95% CI 1.52–1.64). Associations were stronger for premenopausal women. The WQS index was associated with density overall (OR = 1.22, 95% CI 1.20–1.24); the most heavily weighted air toxics were lead and cobalt.
Conclusions
In this first study to evaluate the association between air toxics and breast density, women living in areas with higher concentrations of lead and cobalt were more likely to have dense breasts.
Similar content being viewed by others
Background
Breast density, a marker of heightened breast cancer risk, may be influenced by environmental insults [1]. Women in the highest category of density tend to have a four to fivefold higher risk of breast cancer [2]. Elucidating the role the environment plays in breast cancer is an important area of research, as the incidence of breast cancer remains high [3] and most identified risk factors for breast cancer are not modifiable [4]. Evaluating environmental predictors of breast density may provide more proximal evidence to support a role of chemicals in carcinogenesis and also suggest potential biologic mechanisms of importance.
Although genetics is an important determinant of breast density [5], accumulating evidence suggests that lifestyle and environmental factors, such as hormone therapy (HT) use and cigarette smoking [2, 6,7,8,9], may also influence breast density. Previous studies have considered the relationship between air pollution and other environmental chemicals and breast density with inconsistent results [1, 10,11,12,13,14]. Breast density declines after discontinuing HT or with tamoxifen use [15,16,17], underscoring the modifiability of this risk factor and the potential value of identifying environmental determinants of density.
Both polycyclic aromatic hydrocarbons (PAHs) and heavy metals are environmental endocrine disruptors and can induce oxidative stress that may influence the risk of breast cancer. Sources of PAH exposure [18], including exposure to traffic pollution [19], have been previously related to breast cancer risk, and studies of air toxics have also suggested a role for airborne metals in breast cancer [20, 21]. PAHs are ubiquitous environmental contaminants formed by the combustion of organic material. PAHs have been shown to exhibit both estrogenic and anti-estrogenic effects [22]. In addition, evidence from experimental studies supports the hypothesis that many toxic metals may act as endocrine disruptors, and as such, they are often referred to as “metalloestrogens” [23]. Given these biologic mechanisms, it is plausible that these exposures may also impact breast density.
The aim of this study was to evaluate the relationship between living in areas of higher airborne metals and PAHs and breast density, with exposures assessed both individually, and using a mixture approach. We hypothesized that women residing in an environment with higher levels of air toxics would be more likely to have dense breasts.
Methods
Study population
This study utilized the National Cancer Institute’s Breast Cancer Surveillance Consortium (BCSC) [24]. The BCSC is a collaborative network of mammography registries (www.bcsc-research.org). Five registries provided data for the study: Carolina Mammography Registry, Vermont Breast Cancer Surveillance System, Kaiser Permanente Washington Registry (Washington State), San Francisco Mammography Registry, and New Hampshire Mammography Network. This resource was designed to assess breast cancer screening and patient outcomes from a geographically diverse sample of over 100 community radiology facilities. Women undergoing mammograms at these facilities complete a health questionnaire at each breast imaging exam, which includes items on sociodemographic characteristics, medical history, and breast cancer risk factors. Distributions of sociodemographic characteristics and race in women in BCSC counties are similar to those of the US population [24]. Each registry and the Statistical Coordinating Center (SCC) received institutional review board approval for either passive or active consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act (HIPAA) compliant, and all registries and the SCC have received a federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities who are subjects of this research.
Since 1994, the BCSC has prospectively collected data on mammograms conducted at participating radiology facilities. Breast density information was recorded in clinical practice by the interpreting radiologist using the standard clinical scoring information as determined by the interpreting radiologist using Breast Imaging–Reporting and Data System (BI-RADS) categories (1, entirely fatty; 2, scattered areas of fibroglandular density; 3, heterogeneously dense; 4, extremely dense). Available data elements include demographic characteristics (zip code, age, race, and education), reproductive characteristics (parity), health history (family and personal history of breast cancer), screening mammography history, use of HT, and menopausal status. Zip code level data on income, poverty, and education was derived from the US Census.
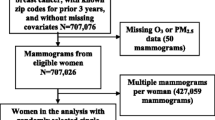
The current analysis was limited to women with no personal history of breast cancer who underwent a routine screening mammogram in 2011 and who were not missing either breast density or residential zip code information (n = 285,817 women). If a woman had more than one mammogram in 2011, we selected the first mammogram of that calendar year.
Exposure assessment
Levels of airborne toxics were assessed using the Environmental Protection Agency (EPA) National Air Toxics Assessment (NATA), a database that provides information on concentrations of toxic air pollutants nationally. The 2011 NATA is the most recently released version of the data. NATA assessed levels of air toxics by using validated air pollution models that utilizes input from the National Emissions Inventory, a comprehensive compilation of information on major stationary sources (factories, incinerators), area and other sources (dry cleaners, small manufacturers), on-road and non-road mobile sources (cars, trucks and boats), events (wildfires), and biogenics (naturally occurring emissions). The validated air pollution model also incorporates secondary information, specifically the formation of secondary pollutants from reactions between pollutants, and background exposure levels from long-range transport from distant sources [25].
NATA provides census-tract level estimates for polycyclic organic matter, which is predominately composed of PAH [26] in addition to PAH derivatives [27], and for the metals arsenic, cadmium, chromium, cobalt, lead, manganese, mercury, nickel, and selenium. BCSC collects information on residential zip code at the time of the mammogram. To link the BCSC data with the NATA exposure information, we used the US Department of Housing and Urban Development zip code crosswalk files to link the census track airborne toxic estimates to the residential zip code [28].
Statistical analysis
The primary analysis was a cross-sectional study to estimate the association between living in a zip code with higher exposure to individual airborne toxics and breast density in 2011. Air toxic exposure levels were categorized based on quartiles, and the same cut points were used consistently throughout all analyses except for a sensitivity analysis for select air toxics in which they were characterized using deciles. Breast density was classified using the BI-RADS categories. Descriptive characteristics and Pearson correlation coefficients (r) were estimated. Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CI) when BI-RADS categories were collapsed to a dichotomous variable with BI-RADS 1 or 2 classified as non-dense and BIRADS 3 or 4 as dense. Multinomial regression was used when the outcome was the four-level BI-RADS categories.
We evaluated effect measure modification of the association between air toxics and breast density by menopausal status and hormone therapy use by including a cross-product term in the model and testing the statistical significance using a likelihood ratio test. We also considered whether PAH or selenium exposure modified the association between the remaining airborne metals and density. These specific exposures were selected as potential effect measure modifiers because selenium has been hypothesized to counteract the negative impacts of metals such as cadmium [29] and PAHs have been hypothesized to act synergistically with toxic metals via induction of oxidative stress [30]. The confounder adjustment set was based on consideration of directed acyclic graphs [31] and included age (≤ 45, 46–50, 51–55, 61–65, 66–69, ≥ 70), race/ethnicity (white, black, Asian/native Hawaiian/Pacific Islander, Hispanic, other), parity (ever/never), and zip code level income (< $50,000, $50,000–$99,999, ≥ $100,000), and education. We did a complete case analysis, limiting to women who did not have missing values for the variables in the adjustment set (n = 222,581 women).
In sensitivity analyses, we considered further covariate adjustment for rural/urban status, hormone replacement therapy, and BMI. BMI data were not collected at all study sites, and BMI was missing for many women (45%). Therefore, we examined the impact of adjusting for BMI as a confounder in analyses limited to BCSC participants with non-missing BMI. We additionally tested whether BMI was correlated with air toxic levels using Pearson correlation statistics.
As a secondary analysis, we used weighted quantile sum (WQS) regression to evaluate the relationship between the air toxic mixture and breast density overall. WQS has been described previously [32,33,34]. In this application, WQS was used to estimate a weighted linear index to evaluate the combined association of correlated air toxics classified in quartiles in relation to breast density. The data were randomly split into a training (40%) and validation (60%) dataset. The weights were empirically determined in the training dataset via bootstrap sampling (n = 100). In WQS, weights can range between 0 and 1 but are constrained to sum to 1 across the individual components of the mixture. If all air toxics received equal weights, the weight for each would be 0.1. Weights greater than 0.1 signify a higher contribution to the weighted index than expected; higher weights indicate stronger associations with breast density. The strengths of WQS include the estimation of an overall mixture effect as well as the identification of the exposures that appear to drive the association. WQS was selected as prior simulation studies have shown it to have a good sensitivity and specificity compared to other mixtures approaches [32, 33].
Results
Of the 222,581 women who met our inclusion criteria, approximately 45% (n = 100,107) had dense breasts, defined as BI-RADS 3 or 4 (Table 1). Women were more likely to fall into the middle BI-RADS categories (2 and 3) than the extremes (1 and 4). As expected, women who were older or postmenopausal, and women with a higher BMI were more likely to have non-dense breasts. Asian women and women who were living in a zip code with a higher median income and education were more likely to have dense breasts.
Quartiles and ranges of exposure to air toxics are shown in Table 2. Median exposure levels were highest for PAH and mercury compared to the other air toxics. The air toxics were moderately correlated (Additional file 1: Table S1, with correlations ranging from − 0.01 to 0.86).
In general, living in areas with higher exposure to some, but not all, individual air toxics was associated with higher odds of having dense breasts (Table 3). Associations were most evident for women who lived in areas with high exposure to cobalt (quartile 4 (Q4) vs. quartile 1(Q1), OR = 1.60, 95% CI 1.56–1.64) and lead (Q4 vs Q1, OR = 1.56, 95% CI 1.52–1.60). Higher odds of dense breasts were also observed for women living in areas with higher exposure to arsenic (Q4 vs Q1, OR = 1.20, 95% CI 1.17–1.23), manganese (Q4 vs Q1, OR = 1.18, 95% CI 1.15–1.21), nickel (Q4 vs Q1, OR = 1.23, 95% CI 1.20–1.26), and PAH (Q4 vs Q1, OR = 1.27, 95% CI 1.23–1.31). Although we observed elevated ORs for the second and third quartiles of chromium and mercury exposure, the associations were not apparent in the fourth quartile. Little to no association was observed for higher quartiles of cadmium or selenium when compared to the lowest quartile. In sensitivity analyses using deciles to evaluate the dose–response trends for cobalt and lead, we found that the observed trends were relatively linear with increasing lead exposure (Additional file 1: Fig. S1). For cobalt, there was an initial inverse trend that then reversed and became positive after the median exposure level.
The relationship between air toxics and breast density differed by menopausal status, with the associations tending to be stronger in women prior to menopause (Table 4). For some of the air toxics (cadmium, chromium, and selenium), there was a positive association with breast density in premenopausal women (e.g., cadmium Q4 vs Q1, OR = 1.26, 95% CI 1.20–1.32) although no positive association was evident in postmenopausal women. For some of the other air toxics (arsenic, cobalt, lead, manganese, nickel, and PAH), a positive association was apparent for both pre- and postmenopausal women but was more pronounced in premenopausal women (e.g., lead, premenopausal Q4 vs Q1, OR = 1.84, 95% CI 1.76–1.93; postmenopausal OR = 1.45, 95% CI 1.41–1.50).
Associations were similar when we classified density using all four BI-RADS categories with associations most pronounced for exposure to arsenic, cobalt, and lead (Additional file 1: Table S2). There was evidence of effect measure modification by HT use for some of the metals, including lead and nickel (Additional file 1: Table S3). For example, the association with lead was apparent in women who were non-users of HT (OR = 1.60, 95% CI 1.56–1.66) but less pronounced in women who reported using HT (OR = 1.10, 95% 0.97–1.25). When considering potential effect measure modification by PAHs or selenium levels, we observed that some associations with density tended to be more pronounced in women who lived in areas below the median levels of PAHs or selenium levels (Additional file 1: Tables S4 and S5).
Further adjustment for rural/urban status and hormone replacement therapy did not substantially alter our results (data not shown). In analyses limited to women with non-missing BMI (which necessitated dropping registries that did not collect BMI), associations between air toxic levels and breast density were similar in models with and without adjustment for BMI, suggesting that even though BMI is related to breast density, it was not influential as a confounder in these analyses (Additional file 1: Table S6). Air toxic levels were not correlated with BMI (data not shown, all r < 0.15).
The WQS index was associated with breast density (OR = 1.22, 95% CI = 1.20, 1.24). A quartile increase in the WQS index resulted in a 20% higher odds of having dense breasts. Only two air toxics contributed meaningfully to the overall effect. These were lead (weight = 0.56) and cobalt (weight = 0.44).
Discussion
In this large study population, we observed that living in areas of higher exposure to certain air toxics, especially lead and cobalt, was associated with higher odds of having dense breasts, particularly in premenopausal women. To the best of our knowledge, this is the first study to consider the relationship between air toxics and breast density. Breast density is a strong risk factor for breast cancer [2]; our findings suggest a role for environmental exposures in breast density and imply a possibly remediable role of these compounds in breast carcinogenesis.
Air pollution has been increasingly shown to be relevant for breast cancer [35]. Traffic-related pollution such as nitrogen dioxide (NO2) and PAH exposure has been suggestively related to breast cancer [19, 36,37,38,39]. Evidence is less consistent for hazardous air toxics [21, 40, 41] or particulate matter [36, 42, 43]. In the Sister Study cohort, living in areas of higher airborne cadmium, lead, mercury, and cobalt was related to a higher postmenopausal breast cancer risk [20]. However, few studies have considered the relationship between air pollution and breast density [12,13,14]. A previous study in the BCSC study population found that women with dense breasts had higher exposure to PM2.5, which is a measure of a mixture of many compounds including toxic metals [14]; these results are consistent with the findings presented here. None of these prior studies evaluated the relationship between breast density and metallic air toxics or PAH exposure. Both metals and PAHs are known to induce oxidative stress [30, 44, 45] and to cause endocrine disruption [22, 23, 46]. Both estrogenic activity [5] and oxidative stress [47] are hypothesized mechanisms by which these compounds may influence breast density and, thus, be relevant for breast cancer.
Air pollution is a complex mixture of many types of exposure, and a strength of this study is the inclusion of a mixture analytic approach to better mimic real life exposure to multiple correlated toxics. The use of the WQS method allows for the quantification of an overall effect—we observed 20% higher odds of dense breasts with each increasing quartile of the mixture exposure index. The WQS approach also permits the identification of the “bad actors” in the presence of correlated exposures. Lead and cobalt were identified to be the only weighted compounds of interest, suggesting they may drive the association, a finding that was consistent with the associations observed for those metals in the individual analysis. A limitation of WQS is that it only considers exposures that exhibit their effect in the same direction [33]; however, none of the air toxics exhibited a strong inverse association with density.
In epidemiologic studies, sources of PAH exposure, including outdoor [19, 39, 48] and indoor air pollution [49, 50], and adduct biomarkers of PAH exposure [51, 52], have been related to breast cancer risk. Although PAH exposure was related to breast density in our individual chemical analysis, in the WQS mixture analysis, it was downweighted to zero suggesting the observed association was actually driven by other correlated exposures.
Most of the research on heavy metals and breast cancer risk has focused on the role of cadmium [53]. The findings from these studies have been inconsistent; case-control studies with urinary cadmium measurements have reported consistently strong positive associations [53,54,55,56,57] whereas prospective cohort studies have not observed an association [58, 59]. Only one study evaluated the association between urinary lead and breast cancer risk; this case-control study found no evidence of an association [60]. Arsenic may be related to breast cancer risk in certain subgroups [61], but there is little evidence to date on the other toxic metals such as cobalt.
Inconsistent findings have been reported regarding the relation between urinary cadmium and breast density [10, 11] although a positive association was observed in one study that was limited to premenopausal women [11] consistent with the results reported here. The associations observed in this study tended to be stronger in women who were premenopausal. In our study population, 62% of premenopausal women had dense breasts whereas only 37% of postmenopausal women did. We also found that associations for some of the airborne metals tended to be higher in women who were non-HT users. This is consistent with a hypothesized estrogenic mechanism; women who were using HT may not be as susceptible to endocrine disrupting actions of the airborne toxics.
Airborne exposures are only a single exposure source, and participants may be exposed to these compounds through other sources including their diet, water sources, and tobacco smoke. We were unable to capture these other sources of exposure in this study. The BCSC did not collect information on cigarette smoking history, an important source of both PAHs and metals [18, 62]. Smoking appears to be associated with lower breast density, likely due to its anti-estrogenic effects [8, 9]. Therefore, a limitation of this study is that we could not evaluate whether this association varies based on cigarette smoking status. A limitation of WQS is that it does not identify interactions across exposures. Therefore, we evaluated some possible interactions of a priori interest including whether the associations with the remaining airborne metals and density varied by selenium exposure, as selenium has antioxidant properties [63] and may counteract the toxic effects of metals such as cadmium [29, 64]. The results for interactions with selenium were in line with that conjecture, with associations for many of the metals more pronounced in women who lived in areas below the median. In contrast, although we expected we might observe synergy between PAH exposure and toxic metals [30], the associations with the airborne toxics tended to be higher in those with low PAH exposure. This result could be due to differential residual confounding by other air pollutants, especially as the association between PAH and density observed here appeared to be driven by confounding with other air toxics as assessed by WQS.
We chose to use the most recently available NATA data release, rather than incorporating prior years of exposure because substantial changes have been made in the methodology over the years. The relevant etiologic window for exposures to alter breast density is unknown. This study was consequently cross-sectional by design, although it is likely that estimated 2011 air toxic levels may also represent past exposure. Previous studies have shown that density continues to change, even later in life [65], and that regimen changes in tamoxifen or HT use can alter breast density within 2–3 years [15, 16]. Thus, it is plausible that recent exposures are relevant for breast density. Another limitation of this study is the use of categorical BI-RADS categories rather than continuous measures of breast density. We did not have access to mammographic images and therefore could not use standardized and automated, objective measures of breast density. The BI-RADS categories have been previously shown to only have moderate interrater agreement [66]. For the main analyses, we grouped these categories into dense and non-dense outcomes for ease of interpretation and to ensure relevance for clinical practice. With the four-level categorization, the effects did tend to be stronger for BI-RADS 4 than for 3, so combining the two may obscure some of the effect. Future work in this area should consider the use of continuous, automated measures of breast density.
We adjusted for relevant covariates including race, zip code level education and income, which were important confounders in this study, and conducted sensitivity analyses for other potential confounding factors. Despite this, we cannot rule out the possibility that residual confounding may be present. Previous studies have reported that women in urban locations have elevated breast density compared to rural populations [67]. In sensitivity analyses, our results were consistent after the adjustment for urban/rural status, but further work is needed to better understand the potential relationships between urban/rural status, air pollution, and breast density. Similarly, BMI did not appear to be an important confounder although limiting to women with BMI data did change alter point estimates, likely due to study population differences in those with and without BMI data as BMI was only collected for some study sites.
This study was very well-powered, and the study population is diverse and generalizable. The air toxics were assessed by the EPA using a validated air pollution model. NATA estimates of lead have been previously related to body burden metal measures in children, supporting the validity of their air toxic models [68]. The dosimetry does have limitations, as exposure is estimated at the zip code level rather than individual level and there is likely some misclassification. Similarly, we also have no data on time spent outside, or at work in another zip code environment, use of air filtering devices in the home, window-opening behavior, etc., which factors would cause individual-level exposure variation. However imperfect, this modeled exposure data is the only resource to evaluate this question on a nationwide scale. Nevertheless, the actual effects of well-measured and well-timed exposures may be considerably stronger than what we report here.
Conclusions
In this large, geographically diverse study, we found that women who live in areas of higher exposure to certain air toxics, especially lead and cobalt, were at higher odds of having dense breasts. These results were stronger in premenopausal women. This is the first study to evaluate the relationship between air toxic metals and PAHs in relation to breast density. Understanding the determinants of breast density is important, as women who have dense breasts are at a four to fivefold higher risk of developing breast cancer [2]. Breast cancer remains the most common cancer among women in the USA [3], and a better understanding of its environmental determinants could contribute both to preventative public health measures and to the elucidation of potential biologic mechanisms.
Abbreviations
- BCSC:
-
Breast Cancer Surveillance Consortium
- BI-RADS:
-
Breast Imaging–Reporting and Data System
- BMI:
-
Body mass index
- CI:
-
95% confidence intervals
- EPA:
-
Environmental Protection Agency
- HIPAA:
-
Health Insurance Portability and Accountability Act
- HT:
-
Hormone therapy
- NATA:
-
National Air Toxics Assessment
- NO2 :
-
Nitrogen dioxide
- OR:
-
Odds ratio
- PAHs:
-
Polycyclic aromatic hydrocarbons
- Q:
-
Quartile
- SCC:
-
Statistical Coordinating Center
References
Sprague BL, Trentham-Dietz A, Hedman CJ, Wang J, Hemming JD, Hampton JM, Buist DS, Bowles EJA, Sisney GS, Burnside ES. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer Res. 2013;15(3):R45.
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. 2018;68(1):7–30.
Interagency Breast Cancer and Environment Research Coordinated Committee: Breast cancer and the environment prioritizing prevention. In.; 2013.
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808.
Gapstur SM, Lopez P, Colangelo LA, Wolfman J, Van Horn L, Hendrick RE. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Biomark Prev. 2003;12(10):1074–80.
Martin LJ, Minkin S, Boyd NF. Hormone therapy, mammographic density, and breast cancer risk. Maturitas. 2009;64(1):20–6.
Jacobsen KK, Lynge E, Vejborg I, Tjonneland A, von Euler-Chelpin M, Andersen ZJ. Cigarette smoking and mammographic density in the Danish Diet, Cancer and Health cohort. Cancer Causes Control. 2016;27(2):271–80.
Butler LM, Gold EB, Conroy SM, Crandall CJ, Greendale GA, Oestreicher N, Quesenberry CP Jr, Habel LA. Active, but not passive cigarette smoking was inversely associated with mammographic density. Cancer Causes Control. 2010;21(2):301–11.
Adams SV, Hampton JM, Trentham-Dietz A, Gangnon RE, Shafer MM, Newcomb PA. Urinary cadmium and mammographic density. Epidemiology. 2017;28(1):e6–7.
Adams SV, Newcomb PA, Shafer MM, Atkinson C, Bowles EJA, Newton KM, Lampe JW. Urinary cadmium and mammographic density in premenopausal women. Breast Cancer Res Treat. 2011;128(3):837–44.
DuPre NC, Hart JE, Bertrand KA, Kraft P, Laden F, Tamimi RM. Residential particulate matter and distance to roadways in relation to mammographic density: results from the nNurses’ Health Studies. Breast Cancer Res. 2017;19(1):124.
Huynh S, von Euler-Chelpin M, Raaschou-Nielsen O, Hertel O, Tjonneland A, Lynge E, Vejborg I, Andersen ZJ. Long-term exposure to air pollution and mammographic density in the Danish Diet, Cancer and Health cohort. Environ Health. 2015;14:31.
Yaghjyan L, Arao R, Brokamp C, O'Meara ES, Sprague BL, Ghita G, Ryan P. Association between air pollution and mammographic breast density in the Breast Cancer Surveilance Consortium. Breast Cancer Res. 2017;19(1):36.
Rutter CM, Mandelson MT, Laya MB, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. Jama. 2001;285(2):171–6.
Chow CK, Venzon D, Jones EC, Premkumar A, O’Shaughnessy J, Zujewski J. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomark Prev. 2000;9(9):917–21.
Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–8.
White AJ, Bradshaw PT, Herring AH, Teitelbaum SL, Beyea J, Stellman SD, Steck SE, Mordukhovich I, Eng SM, Engel LS, et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int. 2016;90:185–92.
Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, Richardson DB, Millikan RC, Engel LS, Shantakumar S, et al. Vehicular traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence: the long island breast cancer study project (LIBCSP). Environ Health Perspect. 2016;124(1):30–8.
White AJ, O'Brien KM, Niehoff NM, Carroll R, Sandler DP. Hazardous metallic air toxics and breast cancer risk in a Nationwide Cohort Study. Epidemiology. 2018;30(1):20–8.
Liu R, Nelson DO, Hurley S, Hertz A, Reynolds P. Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: the California Teachers Study. Epidemiology. 2015;26(3):365–73.
Santodonato J. Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: relationship to carcinogenicity. Chemosphere. 1997;34(4):835–48.
Choe S-Y, Kim S-J, Kim H-G, Lee JH, Choi Y, Lee H, Kim Y. Evaluation of estrogenicity of major heavy metals. Sci Total Environ. 2003;312(1):15–21.
Breast Cancer Surveillance Consortium: evaluating screening performance in practice. Available at: http://www.bcsc-research.org/espp.pdf.
Environmental Protection Agency. Technical support document EPA’s 2011 national-scale air toxics assessment. Research Triangle Park, NC: Office of Air Quality P, and Standards; 2015.
Organization WH: WHO guidelines for indoor air quality: selected pollutants. 2010.
Polycyclic Organic Matter [https://www.env.nm.gov/aqb/projects/openburn/CAchemfacts/pom.pdf].
HUD USPS Zip Code Crosswalk Files [https://www.huduser.gov/portal/datasets/usps_crosswalk.html].
Wei XL, He JR, Cen YL, Su Y, Chen LJ, Lin Y, Wu BH, Su FX, Tang LY, Ren ZF. Modified effect of urinary cadmium on breast cancer risk by selenium. Clin Chim Acta. 2015;438:80–5.
Wang T, Feng W, Kuang D, Deng Q, Zhang W, Wang S, He M, Zhang X, Wu T, Guo H. The effects of heavy metals and their interactions with polycyclic aromatic hydrocarbons on the oxidative stress among coke-oven workers. Environ Res. 2015;140:405–13.
Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48.
Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, Hartge P, Ward MH, Wheeler DC. Analysis of environmental chemical mixtures and non-Hodgkin lymphoma risk in the NCI-SEER NHL study. Environ Health Perspect. 2015;123(10):965–70.
Czarnota J, Gennings C, Wheeler DC. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform. 2015;14(Suppl 2):159–71.
Gennings C, Carrico C, Factor-Litvak P, Krigbaum N, Cirillo PM, Cohn BA. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health. 2013;12(1):12–66.
White AJ, Bradshaw PT, Hamra GB. Air pollution and breast cancer: a review. Curr Epidemiol Rep. 2018;5(2):92–100.
Reding KW, Young MT, Szpiro AA, Han CJ, DeRoo LA, Weinberg C, Kaufman JD, Sandler DP. Breast cancer risk in relation to ambient air pollution exposure at residences in the sister study cohort. Cancer Epidemiol Biomark Prev. 2015;24(12):1907–9.
Hystad P, Villeneuve PJ, Goldberg MS, Crouse DL, Johnson K. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: a case-control study. Environ Int. 2015;74:240–8.
Crouse DL, Goldberg MS, Ross NA, Chen H, Labreche F. Postmenopausal breast cancer is associated with exposure to traffic-related air pollution in Montreal, Canada: a case-control study. Environ Health Perspect. 2010;118(11):1578–83.
Bonner MR, Han D, Nie J, Rogerson P, Vena JE, Muti P, Trevisan M, Edge SB, Freudenheim JL. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiol Biomark Prev. 2005;14(1):53–60.
Hart JE, Bertrand KA, DuPre N, James P, Vieira VM, VoPham T, Mittleman MR, Tamimi RM, Laden F. Exposure to hazardous air pollutants and risk of incident breast cancer in the nurses’ health study II. Environ Health. 2018;17(1):28.
Garcia E, Hurley S, Nelson DO, Hertz A, Reynolds P. Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environ Health. 2015;14(14):14.
Andersen ZJ, Ravnskjær L, Andersen KK, Loft S, Brandt J, Becker T, Ketzel M, Hertel O, Lynge E, Brauner EV. Long-term exposure to fine particulate matter and breast cancer incidence in the Danish Nurse Cohort Study. Cancer Epidemiol Prev Biomarkers. 2016;26(3):428–30.
Andersen ZJ, Stafoggia M, Weinmayr G, Key T. Long-term exposure to ambient air pollution and incidence of postmenopausal breast Cancer in 15 European cohorts within the ESCAPE project; 2017.
Bibi M, Hashmi MZ, Malik RN. The level and distribution of heavy metals and changes in oxidative stress indices in humans from Lahore district, Pakistan. Hum Exp Toxicol. 2016;35(1):78–90.
Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1(6):529–39.
Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut. 2016;213:809–24.
Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10(1):1.
Nie J, Beyea J, Bonner MR, Han D, Vena JE, Rogerson P, Vito D, Muti P, Trevisan M, Edge SB, et al. Exposure to traffic emissions throughout life and risk of breast cancer: the Western New York Exposures and Breast Cancer (WEB) study. Cancer Causes Control. 2007;18(9):947–55.
White AJ, Sandler DP. Indoor wood-burning stove and fireplace use and breast cancer in a prospective cohort study. Environ Health Perspect. 2017;125(7):077011.
White AJ, Teitelbaum SL, Stellman SD, Beyea J, Steck SE, Mordukhovich I, McCarty KM, Ahn J, Rossner P Jr, Santella RM, et al. Indoor air pollution exposure from use of indoor stoves and fireplaces in association with breast cancer: a case-control study. Environ Health. 2014;13(108):13–108.
Shen J, Liao Y, Hopper JL, Goldberg M, Santella RM, Terry MB. Dependence of cancer risk from environmental exposures on underlying genetic susceptibility: an illustration with polycyclic aromatic hydrocarbons and breast cancer. Br J Cancer. 2017;116(9):1229–33.
Gammon MD, Sagiv SK, Eng SM, Shantakumar S, Gaudet MM, Teitelbaum SL, Britton JA, Terry MB, Wang LW, Wang Q, et al. Polycyclic aromatic hydrocarbon-DNA adducts and breast cancer: a pooled analysis. Arch Environ Health. 2004;59(12):640–9.
Larsson SC, Orsini N, Wolk A. Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am J Epidemiol. 2015;182(5):375–80.
Strumylaite L, Kregzdyte R, Bogusevicius A, Poskiene L, Baranauskiene D, Pranys D. Association between cadmium and breast cancer risk according to estrogen receptor and human epidermal growth factor receptor 2: epidemiological evidence. Breast Cancer Res Treat. 2014;145(1):225–32.
Nagata C, Nagao Y, Nakamura K, Wada K, Tamai Y, Tsuji M, Yamamoto S, Kashiki Y. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat. 2013;138(1):235–9.
Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk. Aging. 2010;2(11):804–14.
McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98(12):869–73.
Eriksen KT, McElroy JA, Harrington JM, Levine KE, Pedersen C, Sorensen M, Tjonneland A, Meliker JR, Raaschou-Nielsen O. Urinary cadmium and breast cancer: a prospective Danish Cohort Study. J Nat Cancer Inst. 2016;109(2):djw204.
Adams SV, Shafer MM, Bonner MR, LaCroix AZ, Manson JE, Meliker JR, Neuhouser ML, Newcomb PA. Urinary cadmium and risk of invasive breast cancer in the women's health initiative. Am J Epidemiol. 2016;183(9):815–23.
McElroy JA, Shafer MM, Gangnon RE, Crouch LA, Newcomb PA. Urinary lead exposure and breast cancer risk in a population-based case-control study. Cancer Epidemiol Biomark Prev. 2008;17(9):2311–7.
Khanjani N, Jafarnejad AB, Tavakkoli L. Arsenic and breast cancer: a systematic review of epidemiologic studies. Rev Environ Health. 2017;32(3):267–77.
Bernhard D, Rossmann A, Wick G. Metals in cigarette smoke. IUBMB Life. 2005;57(12):805–9.
Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2008;13(2):102–8.
Al-Waeli A, Pappas AC, Zoidis E, Georgiou CA, Fegeros K, Zervas G. The role of selenium in cadmium toxicity: interactions with essential and toxic elements. Br Poult Sci. 2012;53(6):817–27.
Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, Kerlikowske K, Miglioretti DL. Prevalence of mammographically dense breasts in the United States. J Nat Cancer Inst. 2014;106(10):dju255.
Sprague BL, Conant EF, Onega T, Garcia MP, Beaber EF, Herschorn SD, Lehman CD, Tosteson AN, Lacson R, Schnall MD, et al. Variation in mammographic breast density assessments among radiologists in clinical practice: a multicenter observational study. Ann Intern Med. 2016;19(10):M15–2934.
Viel J-F, Rymzhanova R. Mammographic density and urbanization: a population-based screening study. J Med Screen. 2012;19(1):20–5.
Benson SM, Talbott EO, Brink LL, Wu C, Sharma RK, Marsh GM. Environmental lead and childhood blood lead levels in US children: NHANES, 1999–2006. Arch Environ Occup Health. 2017;72(2):70–8.
Acknowledgements
This study was funded in part by the intramural program of the NIH, and National Institute of Environmental Health Sciences [Z01-ES044005]. The data collection by the Breast Cancer Surveillance Consortium was supported by the National Cancer Institute (P01 CA154292, U54CA163303). The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources please see http://www.bcsc-research.org/work/acknowledgement.html. We thank the BCSC investigators, participating women, mammography facilities, and radiologists for the data they have provided for this study.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
AJW conceived and designed the study, conducted statistical analyses, interpreted results, and wrote and revised the paper. ESO supervised the data acquisition and merging. All authors (AJW, CRW, DPS, BLS, and ESO) contributed to study design, interpretation of results and read and contributed to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Each registry and the Statistical Coordinating Center (SCC) received institutional review board approval for either passive or active consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act (HIPAA) compliant and all registries and the SCC have received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities who are subjects of this research.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Deciles of selected air toxics and breast density, Breast Cancer Surveillance Consortium, 2011. Table S1. Pearson correlation coefficients for air toxics, Breast Cancer Surveillance Consortium, 2011. Table S2. Air toxics and BI-RADS score, Breast Cancer Surveillance Consortium, 2011. Table S3. Air toxics and breast density by hormone therapy use (HT), Breast Cancer Surveillance Consortium, 2011. Table S4. Metallic air toxics and breast density by PAHs, Breast Cancer Surveillance Consortium, 2011. Table S5. Metallic air toxics and breast density by selenium, Breast Cancer Surveillance Consortium, 2011. Table S6. Air toxics and breast density when limited to participants with non-missing body mass index, Breast Cancer Surveillance Consortium, 2011. (DOCX 83 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
White, A.J., Weinberg, C.R., O’Meara, E.S. et al. Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res 21, 24 (2019). https://doi.org/10.1186/s13058-019-1110-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-019-1110-7