Abstract
Biological invasions are a major stressor on ecosystems worldwide, but tools to predict their predatory impact remain limited. Here, we quantified invader impacts using two complementary approaches: functional responses (to reveal per capita and multiple predator interaction strengths) and ecomorphology (to reveal trophic profiles and competitive overlap). We compared Mozambique tilapia Oreochromis mossambicus, a native southern African cichlid, and a near-trophically analogous invasive congener, the Nile tilapia Oreochromis niloticus. Both Nile tilapia and Mozambique tilapia exhibited a potentially prey population destabilizing Type II functional response. In both single and multiple predator pairings, invasive Nile tilapia had significantly greater prey consumption rates than native Mozambique tilapia, and thereby a greater predatory impact than its native congeneric. Attack rates were greater for Nile tilapia than Mozambique tilapia, with both species showing more similar handling times and maximum feeding rates. No evidence for multiple predator effects was detected within or between these species, and therefore impacts of both species increased additively in the presence of conspecific or heterospecific competitors. Morphological trait analyses found general differences between these two species, with the invasive Nile tilapia having distinctively larger lower jaw closing force, gill resistance and gill raker length, which facilitated greater feeding capacities over the native species. Trophic profiles predicted using morphological trait differences showed high dietary overlap and served as evidence for potential exploitative competition between the two species. These results reveal superior interaction strengths and ecomorphological trait profiles of an invasive over native species which could influence impact and native species replacement dynamics. Novel applications of functional response and ecomorphology provide complementary insights into predatory and competitive impacts from invasive species, aiding impact prediction across environmental contexts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are globally recognized as one of the main drivers of biodiversity loss (Meyerson et al. 2019; Britton et al. 2023; IPBES 2023). These invasions come with a number of conservation problems, such as genetic pollution of native species through hybridization, competitive exclusion of species with overlapping resources, or predatory removal by the invasive species (Eby et al. 2006; Gozlan 2008; Jackson et al. 2017). Globally, invasive species are also hugely costly to economies (Cuthbert et al. 2021). As such, understanding and predicting factors influencing impacts of invasive species are of great importance for mitigating impacts in both environments and economies.
Although the introduction pathways of invasive species are well studied (Copp et al. 2005), their establishment success is influenced by various environmental factors (Latombe et al. 2017). Particularly important in this regard are biotic interactions between native and invasive species, which can have strong implications for community dynamics (Britton et al. 2023). Invasive species tend to utilize resources more rapidly and efficiently in comparison to native species (Dick et al. 2013; Ricciardi et al. 2013; Alexander et al. 2014; Faria et al. 2023). Invasive species can also affect native species through interference competition dynamics and the levels of resource use overlap between native and invasive species are central in this regard.
Comparative functional responses (FRs) have emerged as a useful approach for assessing how novel consumers in receiving environments may interact with native food resources (Holling 1965; Denny 2014; Dick et al. 2014; Iacarella et al. 2015), and have been used to quantify potential impacts across a range of species (Alexander et al. 2014; Mofu et al. 2019; South et al. 2019). Measuring and comparing FRs aids in predicting and understanding of impacts from invasive predators under different environmental contexts (Alexander et al. 2014; Barrios-O’Neill et al. 2014). Particularly, FRs can be used to measure potential multiple predator effects (MPEs) (Wasserman et al. 2016; Sentis and Boukal 2018), whereby predators interact not only with their prey but also with other predators in their pursuit for mutual resources (Johnson et al. 2009; Wasserman et al. 2016). Some predatory species hinder the feeding of another through interference competition or other mechanisms (Mofu et al. 2019), whereas certain heterospecific or conspecific predator combinations can result in disproportionately increased prey consumption through facilitation (Hebshi et al. 2008). These MPEs can be significant, with implications for community structure and function (Sih et al. 1998).
While FRs can contribute to short-term predictions of invasive species impact, they cannot uncover mechanistic bases for interactions, such as competition between invasive and native species, reducing transferability from laboratory to in-field environments (Griffen 2021; Landi et al. 2022). Feeding-related morphological traits are a complementary comparative approach, aiding the detection of factors driving resource consumption and possible competitive exclusion of native species by invasive species (Luger et al. 2020). Feeding-related morphological traits impact the predatory performance of species (Luger et al. 2020; Sibbing and Nagelkerke 2000) and provide further information on the species’ capacity to search, attack and consume different prey items (Sibbing and Nagelkerke 2000). Any constraints in these morphological traits can have an influence on the feeding performance of a species and thus affect resource use patterns of individuals (Mihalitsis and Bellwood 2017). Therefore, understanding the way in which an invasive species is specialized morphologically in comparison to its native analogues can help inform assessment and management strategies used in determining mechanisms that facilitate invasive species impact (Siddik et al. 2016; Nagelkerke et al. 2018; Luger et al. 2020). Some studies have reported that introduced species that successfully established into new localities differ both morphologically and functionally from the native biota present in recipient ecosystems (Blanchet et al. 2010; Toussaint et al. 2018). Hence, incorporating an assessment of feeding-related morphological trait differences between native and invasive species can provide an indication of morphological features responsible for high resource consumption in invasive species, which may lead to possible extirpation of native species (Luger et al. 2020).
This study focuses on two congeneric Oreochromis species, namely Nile tilapia, Oreochromis niloticus (Linnaeus 1758) and Mozambique tilapia, Oreochromis mossambicus (Peters 1852). Nile tilapia is native to the Nile River Basin, with detrimental impacts in other African regions reported from as early as the early 1950s, such as in Lake Victoria (Zengeya et al. 2015). Since then, it has become a widespread invasive species, given its popularity in aquaculture globally (Martin et al. 2010; Bittencourt et al. 2014; Gu et al. 2014). In southern Africa, many freshwater systems in are now invaded by Nile tilapia (Weyl 2008; Zengeya et al. 2013a). In South Africa, Nile tilapia was first introduced for aquaculture activities, where it escaped and established feral populations in considerable sections of the Limpopo River basin (van der Waal and Bills 2000; Zengeya et al. 2013a, b; Zengeya et al. 2015) and numerous other river systems in warm regions of the country (Weyl 2008). These feral populations have been implicated in decreased native fish abundances and local extinction of native congeners (Zengeya et al. 2013a). One of the native congeners currently at risk of local extinction due to range overlap and genetic pollution through hybridization with Nile tilapia is Mozambique tilapia, a native southern African cichlid (Skelton 2001; Zengeya et al. 2015). Although these two species are known to share habitat and food resources, feeding dynamics within the context of relative impact on prey resources, and competition potential between the species, are lacking. Hence, this study aimed to contrast resource use and morphological traits between the Nile tilapia and Mozambique tilapia to improve understandings of invasion success and impact of the former. In doing so, we develop a methodological base for impact prediction which combined both resource use through FRs and potential competitive interactions through ecomorphological traits.
We hypothesized that (1) the invader exhibits a higher magnitude FR, and (2) the invasive species potentially reduces consumption by the native fish species when they co-occur, through prey risk reduction mechanisms such as interference competition. To achieve this, a multiple predator effect (MPE) comparative FR design was employed, whereby (a) single predators, (b) conspecific pairs of predators, and (c) heterospecific pairs of predators were exposed to a gradient of prey densities. It was further hypothesized that (3) there will be differences in feeding-related morphological traits between these two species, and (4) these differences will place the invasive Nile tilapia at an advantageous position in terms of resource acquisition and consumption.
Materials and methods
Functional response experiments
Species were housed separately in glass tanks at the South African Institute for Aquatic Biodiversity (SAIAB). Each tank contained conspecific specimens and was fitted with a continuous air supply and filtration system. Aerated water was replaced twice per week to maintain high habitat quality. All fishes were size-matched as far as possible with respect to total length within and between species (total length range: Nile tilapia, 76.25–107.54 mm, mean = 86.33 mm; Mozambique tilapia, 76.11–112.8 mm, mean = 90.49 mm), to reduce the influence of size‐related differences on their feeding. We only used juveniles of each species to eliminate effects of sexual dimorphism. Fishes were maintained on a standardized chironomid diet to control for prior experience to prey in a temperature-controlled laboratory (25 °C) and under a 12 h dark and 12 h light regime.
A MPE comparative FR design was employed, whereby (a) single predators, (b) conspecific pairs of predators, and (c) heterospecific pairs of predators were exposed to the full FR experimental design. The experiments were carried out in 20 L buckets filled with 9 L aerated water. Twenty-four hours prior to the experiments, an individual specimen, or multiple (2 specimens: either a conspecific pair or a heterospecific pair) were selected randomly from the holding tanks and each placed in a 20 L bucket according to their allocated experimental group (i.e. single, conspecific pair, heterospecific pair), where they were held without food to allow for standardization for hunger levels. Thawed chironomids (Ocean Nutrition™) were supplied at six densities per bucket (2, 4, 8, 16, 32 or 64), with a minimum of 6 replicates per density for the two different Oreochromis species or multiple predator combinations in the 20 L buckets. Predators were then allowed to feed for two hours. Prey consumption was examined after the two hours of feeding by counting the number of remaining prey. Once the experiments were complete, the fish were removed from the buckets and euthanized using clove oil (400 mg/L) dissolved in ethanol. Fishes were then dissected and the number of chironomid heads present in the gut counted in order to verify the number of prey consumed (this was done only for the intra/interspecific multiple predator scenarios, to determine per capita feeding; see below).
Morphological traits measurements
Twenty-three feeding-related morphological traits were measured to compare the trophic capacities of Mozambique tilapia and Nile tilapia following criteria by Sibbing and Nagelkerke (2000). Specifically, we measured the following traits: anal fin base, anal fin length, body depth, body width, caudal peduncle depth, eye diameter, total length, gill inter raker distance, gill raker length, gill raker profile, gut length, head length, hyoid length, lower jaw length, lower jaw in-lever for closing, lower jaw out-lever, oral gape axis, oral gape height, oral gape width, operculum depth, postlingual organ width, postorbital length, and protrusion length (unit descriptions per Sibbing and Nagelkerke 2000). These morphological traits were then used to characterize the ability of each species to utilize a particular food type as depicted by Sibbing and Nagelkerke (2000). Measurements of body dimensions of fish were recorded (to the nearest 0.1 mm) using electrical calipers. The length and distance of gill rakers were measured (to the nearest 0.1 mm) under a dissecting microscope using an eyepiece graticule.
Statistical analysis
All statistical analyses were conducted using R version 4.1.2 (R Core Team 2021). Underlying raw data for both FR and morphology are available in the Supplementary Material.
Functional responses
Generalized linear modelling (GLM) was used to categorize functional response (FR) types for the single predator experiments (Juliano 2001; Pritchard et al. 2017) and for categorizing FRs as depicted by the gut contents of Nile tilapia and Mozambique tilapia in the heterospecific pair experiments. The Type II FR is shown by a significantly negative linear coefficient as a response to increasing prey density, whereas Type III FRs are shown by a significantly positive first order term and significantly negative second order term. In the case of prey not being replaced after consumption during the experiment, Rogers’ random predator equation was used to model FRs (Rogers 1972):
where Ne is the number of prey eaten, N0 is the initial prey density supplied, a is the attack constant, h is the handling time and T is the total experimental period. The Lambert W function was used to fit the model to the data (Bolker 2008; Pritchard et al. 2017). Roger’s random predator equation is robust to prey depletion in parameter estimation (Cuthbert et al. 2020).
In the instance that there was no significant evidence for Type II or Type III FR, a flexible version of the FR model was fitted on the data which included the scaling exponent q (Real 1977; Pritchard et al. 2017):
where Ne is the number of prey eaten, N0 is the initial prey density supplied, b is the search coefficient (analogous to attack rate a), q is the scaling coefficient, h is the handing time and T is duration of the experiment. Whereas a Type II FR is analogous to q being fixed at 0, FRs are increasingly sigmoidal (i.e. approaching Type III) where q > 0. Using Akaike’s information criterion (AIC; where lower values depict a better fit), fits from Rogers’ random predator Eq. (1) were compared to flexible FR models (2) with q free vary (0–1) and with q fixed at 1 (analogous to a Type III FR), to select the best model which minimized information loss. The final FR models were bootstrapped 2000 times to generate unbiased 95% confidence intervals (Pritchard et al. 2017).
In order to predict the multiple predator feeding rates, the attack rate (a) and handling time (h) estimates from single predator (i.e. in the absence of conspecifics or heterospecifics) FRs (Eq. 1) were used. These were then compared to the observed multiple predator feeding rates. This was done separately for multiple predator groups (i.e. Nile tilapia + Nile tilapia; Mozambique tilapia + Mozambique tilapia and Nile tilapia + Mozambique tilapia) using the corresponding single predator FR parameters. Predictions of FRs were calculated following McCoy et al. (2012) and Sentis and Boukal (2018):
where N is the prey population density, P is the predator population density, a is the attack rate and h is the handling time obtained from the single predator FR estimates. This model assumes no emergent multiple predator effects and its predictions can be compared to actual (observed) multiple predator feeding trials in order to determine the sign and strength of mutiple predator effects. To generate predictions of expected prey survival from the multiple predator experiments, initial values of N and P were set at the experimental initial prey and predator densities corresponding to the experimental treatment. For each predator treatment and prey density, Eq. (3) was integrated over the full experimental time to get the expected number of surviving prey.
In order to estimate variances around predictions, a global sensitivity analysis that uses the 95% confidence intervals of each FR parameter estimate and their variance–covariance matrix (covariance is assumed to be zero when unknown) to generate 100 random parameter sets using a Latin hypercube sampling algorithm was employed (Soetaert and Petzoldt 2010). For each parameter set (n = 100), Eq. (3) was then integrated over time and expected prey survival calculated using the ‘sensRange’ function in the R package ‘FME’ (Soetaert and Petzoldt 2010). Confidence intervals between predicted and observed FRs were compared to dictate differences (i.e. multiple predator effects) across prey densities.
Morphology and trophic profiles
The food-fish model as proposed by Sibbing and Nagelkerke (2000) was used to extrapolate the type of aquatic food each species will feed on based on their feeding-morphological traits. Firstly, the effects of the measured morphological traits on the capacity to consume a suite of aquatic food resources were established and expressed as positive, negative or zero values (ranging between − 2 and + 2). Values of ± 2 showed strong effects and zero values showed no evidence of the morphological trait having an effect. These combined values from all effects formed a hypothetical food specialist profile, which translated to an ideal relative size of the morphological trait to consume a specific aquatic food resource. Secondly, this hypothetical food profile was then correlated with the measured morphological trait of each species using Kendall’s tau correlation, which resulted in correlation coefficients considered as trophic profiles and regarded as a measure of the predicted capacity of the fish to feed on the different aquatic food resources. Both the values measured from the morphological traits and from the food specialist profile were standardized by subtracting the mean value and dividing by the standard deviation of each variable, which resulted in a mean value of zero and standard deviation of one for the different measured variables to ensure all variables have equal weight (Nagelkerke et al. 2018; Luger et al. 2020).
Lastly, a multivariate principal component analysis (PCA) was done on the standardized traits to compare morphology with trophic profiles to assess the capacity of each species to exploit different food resources. Mean trophic profiles for each species were calculated and clustered by species to explore differences in feeding capacities between the two species, and by food type to explore which food types are likely to differentiate most between them. Clustering was performed using the pvclust package and 10 000 bootstraps.
Results
Single predators
Nile tilapia exhibited significant evidence for a Type II FR, owing to a significant negative first order term (GLM: estimate = − 0.007, z = − 2.796, p = 0.005). Mozambique tilapia did not show any statistically clear evidence for a particular FR, although feeding rates tended to decrease with increasing prey density (GLM: estimate = − 0.003, z = − 1.050, p = 0.294). The Type II FR minimized information loss compared to the flexible FR model irrespective of the scaling exponent q for Mozambique tilapia (i.e. lower AIC). Both species can therefore be categorized as displaying this Type II FR form.
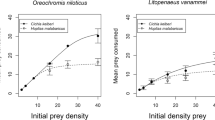
Significant FR parameters were returned for Nile tilapia attack rates and handling times (a: estimate = 0.660, z = 10.999, p < 0.001; h: estimate = 0.012, z = 2.912, p = 0.004) and attack rates but not handling times for Mozambique tilapia (a: estimate = 0.290, z = 8.053, p < 0.001; h: estimate = 0.012, z = 1.091, p = 0.275). Attack rates were therefore substantially greater in Nile tilapia compared to Mozambique tilapia, whereas handling times were similar between species (notwithstanding a lack of FR asymptote at high prey densities). Across all available prey densities, FRs of Nile tilapia were significantly greater in magnitude than that of Mozambique tilapia, as evidenced by a lack of overlap between 95% confidence intervals (Fig. 1a).
Type II functional responses of Nile tilapia and Mozambique tilapia in a single species predator treatments (observed) and b heterospecific pairs based on gut contents. Lines represent fits from the random predator equation, shaded areas are 95 % confidence intervals from non-parametric bootstrapping
Heterospecific pairs
Neither Nile tilapia (GLM: estimate = − 0.006, z = − 1.823, p = 0.068) nor Mozambique tilapia (GLM: estimate = − 0.002, z = − 0.552, p = 0.581) exhibited significant evidence for a Type II FR based on gut contents individually within heterospecific pairs. For both predators, however, the Type II FR fit had the lowest AIC compared to the flexible form of FR considering scaling exponent q.
Significant FR parameters were returned for Nile tilapia (a: estimate = 0.599, z = 7.408, p < 0.001; h: estimate = 0.013, z = 1.972, p = 0.049), but only for the attack rate and not handling time considering Mozambique tilapia (a: estimate = 0.312, z = 6.410, p < 0.001; h: estimate = 0.007, z = 0.547, p = 0.585). Attack rates were accordingly greater in Nile tilapia, whereas handling times were shorter for Mozambique tilapia. Nevertheless, across all prey densities, FRs of Nile tilapia were again significantly higher than Mozambique tilapia (Fig. 1b), with some evidence for convergence at the highest density.
Multiple predator effects
For both conspecific pairs of Nile tilapia and Mozambique tilapia, observed (i.e. actual models from conspecifics) and predicted (i.e. expected models of conspecifics, simulated from individuals) FRs were statistically similar, owing to overlapping 95% confidence intervals (Fig. 2a, b). Conspecific Nile tilapia consumed generally more prey than Mozambique tilapia based on either observations or predictions (Fig. 2a, b).
Experimentally-observed and simulated Type II functional responses of a conspecific Nile tilapia, b conspecific Mozambique tilapia and c heterospecific Nile tilapia and Mozambique tilapia. Lines indicate functional response fits and shaded areas are 95 % confidence intervals. Observed functional responses were empirically recorded, whereas predicted functional responses were simulated from single-species predator attack rate and handling time parameters
For heterospecific pairs of Nile tilapia and Mozambique tilapia, observed FRs again were similar to predicted FRs, given the overlapping of 95% confidence intervals (Fig. 2c). Accordingly, multiple predator effects (MPE) were not significantly evidenced in any intraspecific or heterospecific fish pairing.
Morphology and trophic profiles
The PCA of the morphological trait variables explained only 44.5% of variance of the total variation along the axis of the first two dimensions of the PCA. The PCA did, however, show considerable overlap between Nile tilapia and Mozambique tilapia in the morphological trait, with Nile tilapia more on the right half of the ordination and Mozambique tilapia more on the left half of the ordination (Fig. 3a). Lower jaw closing force, gill raker length, gill resistance, body depth and operculum area were clearly larger for Nile tilapia (Fig. 3b).
Principal component analysis (PCA) ordination of invasive Nile tilapia and native Mozambique tilapia based on feeding-related morphological features. Markers on a depict Nile tilapia (orange circles) and Mozambique tilapia (green squares) and the arrows on b depict the loadings of the most influential variables on the PC-axes
The PCA of trophic profiles for the two species explained 69.7% of the total variance along the first two axes (Fig. 4a). There was a high dietary overlap between Nile tilapia and Mozambique tilapia, with the invasive Nile tilapia showing greater feeding capacity towards phytoplankton, plants, fish (ambush), fish (pursuit) and larvae, which was evident of the species being omnivorous. Both species had similar feeding capacities towards sessile algae, mollusks and insects, with Mozambique tilapia only having greater feeding capacity towards zooplankton (Fig. 4a, b).
Principal component analysis (PCA) ordination of invasive Nile tilapia and native Mozambique tilapia based on trophic profiles. Markers on a depict Nile tilapia (orange circles) and Mozambique tilapia (green squares) and the arrows on b depict the loadings of the most influential food items on the PC-axes
Discussion
By using both comparative functional morphology and FRs, this study advances impact prediction approaches for biological invasions by quantifying and explaining interaction strengths. In our study system, we provide new insights into similarities and differences between two congeneric species (Nile tilapia and Mozambique tilapia), helping to explain in-field impact and replacement dynamics. Our results showed that at the single predator level, the invasive Nile tilapia had greater attack rates than the native species and tended to have significantly higher FR magnitudes. In conspecific or heterospecific multiple predator experiments, however, neither Nile tilapia nor Mozambique tilapia exhibited synergistic or antagonistic feeding. Therefore, Nile tilapia did not adjust their food intake or interfere with feeding of the native, but still consumed more than the native Mozambique tilapia. Thus, the invasive Nile tilapia was found to have greater predatory potential over its native congener in FR experiments. Morphological differences between the two species further placed the invasive Nile tilapia at an advantageous position in terms of resource acquisition and consumption, as they were found to be more generalist in nature.
Predator–prey interactions and the type of FR affects population stability of species (Holling 1965). The present study showed that the invasive Nile tilapia has better predatory performance per capita than the native Mozambique tilapia, given that the former consumes more prey than native congener (Alexander et al. 2014). Furthermore, the higher magnitude FRs shown by Nile tilapia in conspecific specific pairing experiments suggest that the invasive species does not reduce its food intake in the presence of conspecifics and that the single predator scenarios can be extrapolated to multiple conspecific scenarios. In heterospecific combinations, the presence of Mozambique tilapia also did not have a negative impact on the invasive Nile tilapia, indicating that their impacts could combine additively and enhance overall interspecific impact following invasion (Dickey et al. 2021). This additionally suggests that Mozambique tilapia offers little biotic resistance with regards to potential interference competition considering Nile tilapia presence in a receiving environment. These results strongly suggest that the invasive Nile tilapia might outcompete the native Mozambique tilapia in resource-limited environments. However, in ecosystems with a richer variety of resources, niche partitioning could reduce these trophic impacts owing to differences in dietary preferences, for example between detrivory and carnivory. Since our FR analyses consider pairwise predator–prey interactions, unravelling impact outcomes in more complex communities and how these evolve is a future line of research (Rosenbaum et al. 2024).
Differences in attack rates and handling times highlight how predator-predator interactions are dependent on the amount of prey available (Sun et al. 2018). This study showed that the invasive Nile tilapia, based on attack rates, was more successful in searching and acquiring prey than native Mozambique tilapia, although they had more similar handling times in terms of consuming and digesting their prey (Holling 1959; Jeschke et al. 2004; South and Dick 2017). Overall, these results showed that the invasive Nile tilapia has a greater overall predatory potential than the native Mozambique tilapia in simplified prey communities. These experimental findings help to explain mechanisms behind field observations, where trophic overlap exists between Nile tilapia and Mozambique tilapia where they co-occur, and whereby this overlap has placed the native Mozambique tilapia at risk of localized extinction (Zengeya et al. 2013a, 2013b). Within this overlap, the invasive Nile tilapia was found to have a broader diet utilizing a wide range of food resources (Zengeya et al. 2013a), thus is at an advantageous position over native species that utilize the same food resources. While field surveys on diet and species distribution are useful, they offer limited insight into general mechanisms through which invasive species may threaten native fauna. In this regard, comparative FRs are increasingly prevalent in impact studies, given that they can provide better understanding of biotic resistance, as well as predicting invasive species impacts through trophic interactions (Dick et al. 2017; Cuthbert et al. 2018). We view the combination of dietary preference and interaction strength analyses as a powerful synergistic approach in impact prediction.
Assessment of functional morphology provided additional information on competition potential dynamics between these species that are missing from FR analyses, specifically that there is a high morphological overlap between them which facilitates dietary overlap. We suggest that these advances could similarly be applied to assess other invasive species across taxa and environments. A recent study on dietary overlap between Nile tilapia and native species conducted in the upper Kabompo River in Zambia found that Nile tilapia had high dietary overlap with native species, such as O. macrochir and T. sparrmanii, which showed high interspecific competition potential between these species (Jere 2021). Additional research on the diet of Nile tilapia and native species found similar results, where Nile tilapia shared food resources with native species and consumed more than their native counterparts (Zengeya et al. 2015). Such competition may have implications for food web dynamics and eventually lead to declines in native biota and hence loss of biodiversity (Eloranta et al. 2015; Jere 2021), should the invasive species outcompete native analogues.
Our results predicted that Nile tilapia had the ability to consume a greater diversity of food items, many of which Mozambique tilapia may not be able to handle. This was attributed to Nile tilapia having a distinctively greater lower jaw closing force, gill resistance and gill raker length. These key features have all been shown to be advantageous when it comes to feeding, through facilitating greater levels of predatory potential and omnivory (Cooper et al. 2010; Pease et al. 2015). Greater lower jaw force in Nile tilapia suggests that they can handle hardier invertebrate prey than its native analogue. These differences in morphological traits suggest differences in feeding capacities for these two species, providing opportunity for resource partitioning and for the invasive species to become successfully integrated into the receiving ecosystem (Nagelkerke et al. 2018; Jere 2021). Nile tilapia has been identified as an opportunistic feeder, allowing it to withstand and overcome competition for food resources as it is able to use a wide range of available resources (Winemiller and Kelso‐Winemiller 2003; Jere 2021). The versatility in feeding displayed by the invasive species may play an important role in its establishment success when introduced in the native range of Mozambique tilapia, as it would afford Nile tilapia the opportunity to exploit a broader range of food resources compared to Mozambique tilapia. Dietary overlap and similarities in trophic profiles are imbued from morphological trait similarities between native and invasive species, and these similarities may result in exploitative competition, leading to possible extinction of native species (Nagelkerke et al. 2018; Luger et al. 2020).
Our study, while providing useful information that links FR and morphological traits to ecological impact through predation mechanisms, is subject to several limitations. First, we obtained fish from an aquaculture facility, a source of individuals which may not resemble traits of wild populations. While we thus acknowledge that farmed fish may have different trait profiles within species than wild individuals, we argue that this remains a fair comparison because (i) both species were obtained from the same facility, (ii) aquaculture is a major pathway for the introduction of invasive fish populations, and (iii) commercial sources limit environmental pressures from sampling native populations and ethical concerns. To that end, our comparisons particularly reflect ecological scenarios following immediate escape from aquaculture facilities. A second inherent limitation to our FR experimentation approach is that feeding trials were done in relatively small volumes of water. This limited capacity led to a high prey density per unit volume and likely elevated the FR curve magnitudes, in turn likely contributing to low statistical clarity in handling time estimates for Mozambique tilapia due to prey depletion. This could have been resolved with additional prey densities or greater container volumes, where the curves would be more likely to saturate (Kalinkat et al. 2023). Nevertheless, these feeding conditions were carefully matched between species and across fish densities, allowing for a standardized comparison of trophic interactions as well as phenomenological interpretation of FR parameters. We also notably captured the underlying uncertainty in FR parameters when predicting multiple predator feeding rates, by implementing sensitivity analyses. Thirdly, FRs are relatively short-term measures which do not necessarily inform long-term ecological impacts or from mechanisms other than trophic interactions. Factors such as birth rates and mortality rates are also important demographic components to consider in order to make robust long-term impact predictions, alongside prey switching behaviours and effects through mechanisms such as disease and hybridization (Landi et al. 2022). Arguably the greatest impact that Nile tilapia will have on Mozambique tilapia is through hybridisation. Thus, further research focusing on mitigation strategies for invasive species is needed to conserve native gene pools. Lastly, we acknowledge that body size alongside temperature is a major driver of interaction strengths between species, especially in relative terms between predators and prey (Rall et al. 2012; Cuthbert et al. 2020). While we endeavored to match body size here within and between species, shifts in body size ratios alongside environmental changes in the wild could severely modify trophic interaction networks, translating to altered ecological impact outcomes and trait efficiencies in resource acquisition. As such, care needs to be taken when extrapolating these results to field scenarios.
Conclusions
Similar multi-faceted research approaches can be implemented in future to broaden our understanding of feeding dynamics within the context of invasion potential, which may aid in identifying priority species that require conservation. Combining FRs and ecomorphological approaches could be useful in identifying problematic invasive species and quantifying their potential impacts, which is crucial in prioritizing management actions (Robertson et al. 2020). Feeding dynamics within the context of competition potential are largely lacking for Nile tilapia and Mozambique tilapia, and hence this study provided new information on the trophic interactions of these two species. In addition, their functional morphology had yet to be contrasted, limiting our understanding of how their feeding-related morphological traits may facilitate a competitive advantage between invasive and native species. Our assessment of FRs and morphological differences between these two species showed that increased attack rates and morphological traits allowing for consumption of a broader range of resources are responsible for invasive Nile tilapia having high predatory potential than Mozambique tilapia. The combined use of FRs and ecomorphological analyses in other study systems could allow for enhanced impact prediction from biological invasions.
Data availability
If our manuscript is accepted, data will be made available on the Dryad Digital Repository.
References
Alexander ME, Dick JT, Weyl OL, Robinson TB, Richardson DM (2014) Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol Lett 10:20130946. https://doi.org/10.1098/rsbl.2013.0946
Barrios-O’Neill D, Dick JT, Emmerson MC, Ricciardi A, MacIsaac HJ, Alexander ME, Bovy HC (2014) Fortune favours the bold: a higher predator reduces the impact of a native but not an invasive intermediate predator. J Anim Ecol 83:693–701. https://doi.org/10.1111/1365-2656.12155
Bittencourt LS, Pinheiro DA, Cárdenas MQ, Fernandes BM, Tavares-Dias M (2014) Parasites of native Cichlidae populations and invasive Oreochromis niloticus (Linnaeus, 1758) in tributary of Amazonas River (Brazil). Rev Bras Parasitol Vet 23:44–54. https://doi.org/10.1590/S1984-29612014006
Blanchet S, Grenouillet G, Beauchard O, Tedesco PA, Leprieur F, Dürr HH, Busson F, Oberdoff T, Brosse S (2010) Non-native species disrupt the worldwide patterns of freshwater fish body size: implications for Bergmann’s rule. Ecol Lett 13(4):421–431. https://doi.org/10.1111/j.1461-0248.2009.01432.x
Bolker BM (2008) Ecological models and data in R. Princeton University Press, New Jersey
Britton JR, Lynch AJ, Bardal H, Bradbeer SJ, Coetzee JA, Coughlan NE, Dalu T, Tricarico E, Gallardo B, Lintermans M, Lucy F (2023) Preventing and controlling non-native species invasions to bend the curve of global freshwater biodiversity loss. Environ Rev 31:310–326. https://doi.org/10.1139/er-2022-0103
Cooper WJ, Parsons K, McIntyre A, Kern B, McGee-Moore A, Albertson RC (2010) Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. PLoS ONE 5:e9551. https://doi.org/10.1371/journal.pone.0009551
Copp GH, Garthwaite R, Gozlan RE (2005) Risk identification and assessment of non-native freshwater fishes: a summary of concepts and perspectives on protocols for the UK. J Appl Ichthyol 21:371
Cuthbert RN, Dickey JW, McMorrow C, Laverty C, Dick JT (2018) Resistance is futile: lack of predator switching and a preference for native prey predict the success of an invasive prey species. R Soc Open Sci 5:180339. https://doi.org/10.1098/rsos.180339
Cuthbert RN, Wasserman RJ, Dalu T, Kaiser H, Weyl OL, Dick JT, Sentis A, McCoy MW, Alexander ME (2020) Influence of intra-and interspecific variation in predator–prey body size ratios on trophic interaction strengths. Ecol Evol 10(12):5946–5962. https://doi.org/10.1002/ece3.6332
Cuthbert RN, Pattison Z, Taylor NG, Verbrugge L, Diagne C, Ahmed DA, Courchamp F (2021) Global economic costs of aquatic invasive alien species. Sci Total Environ 775:145238. https://doi.org/10.1016/j.scitotenv.2021.145238
Denny M (2014) Buzz Holling and the functional response. Bull Ecol Soc Am 95:200–203
Dick JTA, Gallagher K, Avlijas S, Clarke HC, Lewis SE, Leung S, Minchin D, Caffrey J, Alexander ME, Maguire C, Harrod C, Reid N, Haddaway NR, Farnsworth KD, Penk M, Ricciardi A (2013) Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol Invasions 15:837–846. https://doi.org/10.1007/s10530-012-0332-8
Dick JT, Alexander ME, Jeschke JM, Ricciardi A, MacIsaac HJ, Robinson TB, Kumschick S, Weyl OL, Dunn AM, Hatcher MJ, Paterson RA (2014) Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol Invasions 16:735–753. https://doi.org/10.1007/s10530-013-0550-8
Dick JT, Alexander ME, Ricciardi A, Laverty C, Downey PO, Xu M, Jeschke JM, Saul WC, Hill MP, Wasserman R, Barrios-O’Neill D (2017) Functional responses can unify invasion ecology. Biol Invasions 19:16675–21672. https://doi.org/10.1007/S10530-016-1355-3
Dickey JW, Coughlan NE, Dick JT, Médoc V, McCard M, Leavitt PR, Lacroix G, Fiorini S, Millot A, Cuthbert RN (2021) Breathing space: deoxygenation of aquatic environments can drive differential ecological impacts across biological invasion stages. Biol Invasions 23(9):2831–2847. https://doi.org/10.1007/s10530-021-02542-3
Eby LA, Roach WJ, Crowder LB, Stanford JA (2006) Effects of stocking-up freshwater food webs. Trends Ecol Evol 21:576–584. https://doi.org/10.1016/j.tree.2006.06.016
Eloranta AP, Nieminen P, Kahilainen KK (2015) Trophic interactions between introduced lake trout (Salvelinus namaycush) and native Arctic charr (S. alpinus) in a large Fennoscandian subarctic lake. Ecol Freshw Fish 24:181–192. https://doi.org/10.1111/eff.12132
Faria L, Cuthbert RN, Dickey JWE, Jeschke JM, Ricciardi A, Dick JTA, Vitule JRS (2023) The rise of the functional response in invasion science: a systematic review. NeoBiota 85:43–79. https://doi.org/10.3897/neobiota.85.98902
Gozlan RE (2008) Introduction of non-native freshwater fish: is it all bad? Fish Fish 9:106–115. https://doi.org/10.1111/j.1467-2979.2007.00267.x
Griffen BD (2021) Considerations when applying the consumer functional response measured under artificial conditions. Front Ecol Evol 9:713147. https://doi.org/10.3389/FEVO.2021.713147
Gu DE, Luo D, Xu M, Ma GM, Mu XD, Luo JR, Hu YC (2014) Species diversity defends against the invasion of Nile tilapia (Oreochromis niloticus). Knowl Manag Aquat Ecosyst 414:07
Hebshi AJ, Duffy DC, Hyrenbach KD (2008) Associations between seabirds and subsurface predators around Oahu. Hawaii Aquat Biol 4:89–98. https://doi.org/10.3354/ab00098
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European Pine Sawfly1. Can Entomol 91:293–320. https://doi.org/10.4039/ENT91293-5
Holling CS (1965) The functional response of predators to prey density and its role in mimicry and population regulation. Memoirs Entomol Soc Can 97:5–60. https://doi.org/10.4039/ENTM9745FV
Iacarella JC, Dick JT, Alexander ME, Ricciardi A (2015) Ecological impacts of invasive alien species along temperature gradients: testing the role of environmental matching. Ecol Appl 25:706–716. https://doi.org/10.1890/14-0545.1
IPBES (2023) Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. In: Roy HE, Pauchard A, Stoett P, Renard Truong T, Bacher S, Galil BS, Hulme PE, Ikeda T, Sankaran KV, McGeoch MA, Meyerson LA, Nuñez MA, Ordonez A, Rahlao SJ, Schwindt E, Seebens H, Sheppard AW, Vandvik V. (eds.) IPBES secretariat, Bonn, Germany
Jackson MC, Wasserman RJ, Grey J, Ricciardi A, Dick JT, Alexander ME (2017) Novel and disrupted trophic links following invasion in freshwater ecosystems. Adv Ecol Res 57:55–97. https://doi.org/10.1016/bs.aecr.2016.10.006
Jere A (2021) Diet overlap and feeding preference of Oreochromis niloticus (Linnaeus, 1758) versus two native cichlids of the upper Kabompo River, northwest of Zambia. Authorea Preprints. https://doi.org/10.22541/au.163898045.52297636
Jeschke JM, Kopp M, Tollrian R (2004) Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol Rev 79:337–349. https://doi.org/10.1017/S1464793103006286
Johnson PTJ, Olden JD, Solomon CT, Vander Zanden MJ (2009) Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159:161–170. https://doi.org/10.1007/s00442-008-1176-x
Juliano SA (2001) Nonlinear curve fitting: predation and functional response curves. Des Anal Ecol Exp 2:178–196
Kalinkat G, Rall BC, Uiterwaal SF, Uszko W (2023) Empirical evidence of type III functional responses and why it remains rare. Front Ecol Evol 11:1033818. https://doi.org/10.3389/fevo.2023.1033818
Landi P, McCoy MW, Vonesh JR (2022) Predicting invasive predator impact via the comparative functional response approach: linking application to ecological theory.
Latombe G, Pyšek P, Jeschke JM, Blackburn TM, Bacher S, Capinha C, Costello MJ, Fernandez M, Gregory RD, Hobern D, Hui C (2017) A vision for global monitoring of biological invasions. Biol Cons 213:295–308. https://doi.org/10.1016/j.biocon.2016.06.013
Luger AM, South J, Alexander ME, Ellender BR, Weyl OLF, Nagelkerke AJ (2020) Ecomorphology of largemouth bass relative to a native trophic analogue explains its high invasive impact. Biol Invasions 22:2223–2233. https://doi.org/10.1007/s10530-020-02252-2
Martin CW, Valentine MM, Valentine JF (2010) Competitive interactions between invasive Nile tilapia and native fish: the potential for altered trophic exchange and modification of food webs. PLoS ONE 5(12):e14395. https://doi.org/10.1371/journal.pone.0014395
McCoy MW, Stier AC, Osenberg CW (2012) Emergent effects of multiple predators on prey survival: the importance of depletion and the functional response. Ecol Lett 15:1449–2145. https://doi.org/10.1111/ele.12005
Meyerson LA, Simberloff D, Boardman L, Lockwood JL (2019) Toward “rules” for studying biological invasions. Bull Ecol Soc Am 100:1–9
Mihalitsis M, Bellwood DR (2017) A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS ONE 12:e0184679. https://doi.org/10.1371/journal.pone.0184679
Mofu L, South J, Wasserman RJ, Dalu T, Woodford DJ, Dick JT, Weyl OL (2019b) Inter-specific differences in invader and native fish functional responses illustrate neutral effects on prey but superior invader competitive ability. Freshw Biol 64:1655–1663. https://doi.org/10.1111/fwb.13361
Nagelkerke LA, van Onselen E, van Kessel N, Leuven RS (2018) Functional feeding traits as predictors of invasive success of alien freshwater fish species using a food-fish model. PLoS ONE 13:e0197636. https://doi.org/10.1371/journal.pone.0197636
Pease AA, Taylor JM, Winemiller KO, King RS (2015) Ecoregional, catchment, and reach-scale environmental factors shape functional-trait structure of stream fish assemblages. Hydrobiologia 753:265–283. https://doi.org/10.1007/s10750-015-2235-z
Pritchard DW, Paterson RA, Bovy HC, Barrios-O’Neill D (2017) Frair: an R package for fitting and comparing consumer functional responses. Methods Ecol Evol 8:1528–1534. https://doi.org/10.1111/2041-210X.12784
R Core Team. (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rall BC, Brose U, Hartvig M, Kalinkat G, Schwarzmüller F, Vucic-Pestic O, Petchey OL (2012) Universal temperature and body-mass scaling of feeding rates. Philos Trans R Soc B Biol Sci 367:2923–2934. https://doi.org/10.1098/rstb.2012.0242
Real LA (1977) The kinetics of functional response. Am Nat 111(978):289–300
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of nonnative species. Ecol Monogr 83:263–282. https://doi.org/10.1890/13-0183.1
Robertson P, Mill A, Novoa A, Jeschke JM, Essl F, Gallardo B (2020) A proposed unified framework to describe the management of biological invasions. Biol Invasions 22:2633–2645. https://doi.org/10.1007/s10530-020-02298-2
Rogers D (1972) Random search and insect population models. J Anim Ecol 369–383
Rosenbaum B, Li J, Hirt MR, Brose U (2024) Towards understanding interactions in a complex world: design and analysis of multi-species functional response experiments. Methods Ecol Evol. https://doi.org/10.1111/2041-210X.14372
Sentis A, Boukal DS (2018) On the use of functional responses to quantify emergent multiple predator effects. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-30244-9
Sibbing FA, Nagelkerke LA (2000) Resource partitioning by Lake Tana barbs predicted from fish morphometrics and prey characteristics. Rev Fish Biol Fish 10:393–437. https://doi.org/10.1023/A:1012270422092
Siddik MA, Hanif M, Chaklader MR, Nahar A, Fotedar R (2016) A multivariate morphometric investigation to delineate stock structure of gangetic whiting, Sillaginopsis panijus (Teleostei: Sillaginidae). Springerplus 5:1–13. https://doi.org/10.1186/s40064-016-2143-3
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13(9):350–355. https://doi.org/10.1016/S0169-5347(98)01437-2
Skelton PH (2001) A complete guide to the freshwater fishes of southern Africa. Struik Publishers, Cape Town, pp 1–395
Soetaert KER, Petzoldt T (2010) Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. J Stat Softw 33(3)
South J, Dick JT (2017) Effects of acute and chronic temperature changes on the functional responses of the dogfish Scyliorhinus canicula (Linnaeus, 1758) towards amphipod prey Echinogammarus marinus (Leach, 1815). Environ Biol Fish 100:1251–1263. https://doi.org/10.1007/s10641-017-0640-z
South J, McCard M, Khosa D, Mofu L, Madzivanzira TC, Dick JT, Weyl OL (2019) The effect of prey identity and substrate type on the functional response of a globally invasive crayfish. NeoBiota 52:9
Sun Y, Wang F, Yang C, Liu D, Wang X, Su X (2018) Predator size influences intraspecific multiple predator effects in swimming crab-Manila clam system. Aquaculture 488:74–79. https://doi.org/10.1016/j.aquaculture.2018.01.029
Toussaint A, Charpin N, Beauchard O, Grenouillet G, Oberdorff T, Tedesco PA, Brosse S, Villéger S (2018) Non-native species led to marked shifts in functional diversity of the world freshwater fish faunas. Ecol Lett 21(11):1649–1659. https://doi.org/10.1111/ele.13141
Van der Waal BCW, Bills R (2000) Oreochromis niloticus (Teleostei: Cichlidae) now in the Limpopo River system. S Afr J Sci 96:47–48. https://doi.org/10.10520/AJA00382353_7721
Wasserman RJ, Alexander ME, Dalu T, Ellender BR, Kaiser H, Weyl OL (2016) Using functional responses to quantify interaction effects among predators. Funct Ecol 30:1988–1998. https://doi.org/10.1111/1365-2435.12682
Weyl OLF (2008) Rapid invasion of a subtropical lake fishery in central Mozambique by Nile tilapia, Oreochromis niloticus (Pisces: Cichlidae). Aquat Conserv Mar Freshw Ecosyst. https://doi.org/10.1046/j.1095-8649.2003.00134.x
Winemiller KO, Kelso-Winemiller LC (2003) Food habits of tilapiine cichlids of the Upper Zambezi River and floodplain during the descending phase of the hydrologic cycle. J Fish Biol 63:120–128. https://doi.org/10.1046/j.1095-8649.2003.00134.x
Zengeya TA, Robertson MP, Booth AJ, Chimimba CT (2013a) Ecological niche modelling of the invasive potential of Nile tilapia Oreochromis niloticus in African river systems: concerns and implications for the conservation of indigenous congenerics. Biol Invasions 15:1507–1521. https://doi.org/10.1007/s10530-012-0386-7
Zengeya TA, Robertson MP, Booth AJ, Chimimba CT (2013b) A qualitative ecological risk assessment of the invasive Nile tilapia, Oreochromis niloticus in a sub-tropical African river system (Limpopo River, South Africa). Aquat Conserv Mar Freshwat Ecosyst 23:51–64. https://doi.org/10.1002/aqc.2258
Zengeya TA, Booth AJ, Chimimba CT (2015) Broad niche overlap between invasive Nile tilapia Oreochromis niloticus and indigenous congenerics in southern Africa: should we be concerned? Entropy 17:4959–4973. https://doi.org/10.3390/e17074959
Acknowledgements
We acknowledge the late Prof O.L.F. Weyl for initiating this project and dedicate it to his memory. This research was funded by the Department of Science and Innovation-National Research Foundation, South African Research Chairs Initiative in Inland Fisheries and Freshwater Ecology (UID 110507). We acknowledge the use of laboratories and equipment provided by the NRF-SAIAB Research Platform and the Department of Zoology and Entomology at Rhodes University. Lastly, N.P.M. thanks Dr Casey Broom for his assistance and inputs in statistical analysis of the morphological data. R.N.C. is funded by an Early Career Fellowship from the Leverhulme Trust (ECF-2021-001).
Author information
Authors and Affiliations
Contributions
N.P.M., R.J.W., and J.P. contributed equally to the study design; N.P.M. conducted the experiments and data collection; N.P.M., R.N.C., J.P., and R.J.W. led the writing; Data analysis was done by N.P.M. and R.N.C. All authors had equal inputs in the revision of this manuscript and its approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Collection and housing of animals were carried out in compliance with the Department of Environmental Affairs permit no(s) 5073821078112716 and 5078210728122315. Ethical clearance was issued by Rhodes University Animal Research Ethics Committee (RU-AREC ref no. 2021 2695 5969). The fish for this study were sourced from Rivendell fish farm, Eastern Cape.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mpanza, N.P., Cuthbert, R.N., Pegg, J. et al. Assessing biological invasion predatory impacts through interaction strengths and morphological trophic profiling. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03435-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03435-x