Abstract
Thousand cankers disease of Juglans (walnut) and Pterocarya (wingnut) spp. (Fagales: Juglandaceae) is caused by the fungal pathogen Geosmithia morbida Kolarík, Freeland, Utley, and Tisserat (Hypocreales: Bionectriaceae) and bark beetle pest/vector, Pityophthorus juglandis Blackman (Coleoptera: Curculionidae). To further the development of biological management strategies for thousand cankers disease, we assessed the ability of 14 endophytic Trichoderma (Hypocreales: Hypocreaceae) isolates and the commercially available isolate T. afroharzianum strain KRL-AG2 to inhibit the in vitro growth of three different G. morbida isolates via mycoparasitism and antibiosis. To identify factors that may affect field success of candidate biological control agents, we quantified the growth responses of Trichoderma spp. and the commercially available entomopathogenic fungus, Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) strain GHA, to the plant secondary metabolite and antimicrobial compound, juglone in vitro. A total of 12 Trichoderma isolates (from six different Trichoderma species) demonstrated antagonistic activity towards G. morbida in dual-plate assays. Juglone consistently reduced the growth of B. bassiana strain GHA and 14 of the 15 screened Trichoderma isolates in vitro. Additionally, one metabolite-producing Trichoderma isolate, TN4-47, completely inhibited the growth of all three G. morbida isolates across all tested metabolite concentrations and had comparatively greater tolerance to juglone compared to other Trichoderma isolates. Future lines of research should focus on characterizing the active antagonistic compound present in the metabolite filtrates, determine the mode of action of the active component(s), and elucidate how abiotic and biotic factors may influence the growth, persistence, and antagonistic activity of candidate biological control agents in planta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of biological control agents (BCAs) for management of forest pests and pathogens is gaining traction as forest health declines globally (Rabiey et al. 2019; Halecker et al. 2020; Prospero et al. 2021). Biological control strategies are considered to pose a lower environmental risk compared to conventional chemical pesticides (Rabiey et al. 2019; Prospero et al. 2021). Additionally, when considering diseases of nut-bearing crops such as thousand cankers disease (TCD) of walnut (Juglans spp.) and wingnut (Pterocarya spp.) trees, options for pesticide usage are limited due to food safety concerns (Tisserat et al. 2009). Scarcity of effective management strategies for TCD has made the protection of walnut trees from the insect vector/pest, the walnut twig beetle (WTB; Pityophthorus juglandis) Blackman (Coleoptera: Curculionidae) and the pathogenic fungus, Geosmithia morbida Kolarík, Freeland, Utley, and Tisserat (Hypocreales: Bionectriaceae) challenging (Tisserat et al. 2009; Kolařík et al. 2011).
Candidate entomopathogens for management of TCD include Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) strain GHA, a commercial strain that induced mortality of WTB in vitro (Castrillo et al. 2017, Mayfield et al. 2019). However, field efficacy remains doubtful, as applications reduced brood emergence in only one of three trials (Castrillo et al. 2017, Mayfield et al. 2019). Another potential approach for the biological management of TCD is to use host-associated fungal endophytes (Schulz et al. 2019). A previous culture-dependent cataloging of walnut fungal endophytes recovered Trichoderma isolates from the phloem of J. nigra trees that displayed antagonism towards G. morbida in vitro (Gazis et al. 2018). Isolates of Trichoderma spp. are commonly used as BCAs due to their ability to directly parasitize fungal pathogens (mycoparastism) (Ferreira and Musumeci 2021) and secrete fungistatic and fungicidal secondary metabolites (antibiosis) (Halecker et al. 2020; Martínez-Arias et al. 2021). While the endophytic Trichoderma isolates previously examined in Gazis et al. (2018) demonstrated antagonism towards multiple G. morbida isolates in vitro, we have limited knowledge regarding their ability to produce antifungal secondary metabolites.
One persistent challenge for biological management of TCD is translating in vitro efficacy of BCAs to the field. A potential barrier is the compatibility of candidate BCAs with plant host secondary metabolites (Mann and Davis 2020). Juglans spp. produce a variety of antifungal secondary metabolites, including the phenolic allelochemical juglone (5-hydroxy-1,4,-napthoquinone) (Wianowska et al. 2016). It is currently unknown if previously examined candidate BCAs are affected by juglone. Therefore, to further our understanding of the interactions between endophytic Trichoderma isolates and G. morbida and their potential use as BCAs, we assessed the antagonistic activity of four previously examined Trichoderma isolates and expanded the study to include ten unexamined Trichoderma isolates originally recovered from Juglans spp. trees [descriptions provided in Gazis et al. (2018)] towards G. morbida using dual-plate and antifungal metabolite assays. Additionally, to better understand how J. nigra secondary metabolites interact with the efficacy of candidate BCAs, we quantified the growth responses of these same Trichoderma isolates to juglone. For comparison purposes, we included the commercial strains B. bassiana strain GHA and T. afroharzianum strain KRL-AG2. We hypothesized that endophytic isolates would better tolerate juglone when compared with commercially available broad-spectrum BCAs.
Materials and methods
Fungal materials
Fifteen Trichoderma isolates representing seven species, three G. morbida isolates, and one B. bassiana isolate were used in the study (Table 1; Fig. 1). Isolate identities were confirmed using molecular methods targeting the internal transcribed spacer (ITS) region (White et al. 1990; Cai and Druzhinina 2021). To improve confidence in Trichoderma taxonomic assignments, we also amplified the transcription elongation factor (EF-1α) as described in Cai and Druzhinina (2021). Genomic DNA was extracted from fungal mycelium grown on half-strength potato dextrose agar (PDA; Fisher Scientific, Pittsburgh, PA, USA) using the Thermo Scientific™ GeneJET Genomic DNA Purification kit (Fisher Scientific) (Gazis et al. 2018). For Trichoderma isolates we amplified the ITS region using the ITS5 and ITS4 primers (White et al. 1990) and the EF-1α using the EF1 and EF2 primers (O’Donnell et al. 1998). For G. morbida and B. bassiana, we amplified the ITS region using the ITS1 and ITS4 primers (White et al. 1990). All PCR reactions contained a final volume of 40 μl and consisted of 16 μl of GoTaq®G2 Hot Start Master Mix (Promega Corp., Madison, WI, USA), 4 μl of 10 mM forward primer, 4 μl of 10 mM reverse primer, 2 μl dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA), 10 μl of PCR grade water (Promega Corp.), and 4 μl of template DNA. All PCR reactions were performed using a SimpliAmp™ Thermal Cycler (Fisher Scientific). For the Trichoderma ITS region and EF-1α, thermal cycler settings were as follows: initial denaturation at 95 °C for 3 min, followed by 32 cycles of denaturation at 95 °C for 15 s, primer annealing at 53 °C for 15 s, extension at 72 °C for 1 min, and final extension at 72 °C for 5 min (Cai and Druzhinina 2021). For G. morbida and B. bassiana, thermal cycler settings were as follows: initial denaturation at 94 °C for 2 min, followed by 40 cycles of denaturation at 94 °C for 30 s, primer annealing at 57 °C for 1 min, extension at 72 °C for 1.5 min, and final extension at 72 °C for 7 min. Sanger sequencing of all amplicons was completed by Molecular Cloning Laboratories (San Francisco, CA, USA).
Trichoderma maximum likelihood phylogenetic tree constructed using the fourth large intron of the EF-1α with 1000 bootstraps. Isolates used in the study are bolded. All other sequences were gathered from NCBI GenBank and isolate names are preceded by accession numbers. Nodes with bootstrap support values exceeding 90% are marked with a dot. Branch lengths represent evolutionary distance (i.e., the number of substitutions per site)
Sequence quality assessment and assembly of consensus sequences was completed using Unipro UGENE v.45.1 (Okonechnikov et al. 2012). Species level assignments were completed using the Basic Local Alignment Search Tool (BLAST) against the NCBI nucleotide database (Altschul et al. 1990). For Trichoderma isolates, we used TrichoMark 2020 to trim EF-1α sequences to include only the fourth large intron, a phylogenetic marker used to distinguish between Trichoderma spp. (Dou et al. 2020; Cai and Druzhinina 2021). Additionally, for Trichoderma isolates, BLAST searches were restricted to type material (Cai and Druzhinina 2021). For the Trichoderma isolates, we constructed a maximum likelihood phylogenetic tree using the msa function (method = “ClustalW”) from the msa package to align the trimmed EF-1α sequences (Bodenhofer et al. 2015). Pairwise distances were calculated and a UPGMA tree was constructed using the dist.ml and upgma functions from phangorn (Schliep 2011). Tree likelihood was calculated using the pml function (k = 4) and optimized using optim.pml from phangorn (Schliep 2011). We used bootstrap.pml to construct 1000 bootstrap trees. The final tree was plotted using the ggtree function from ggtree (Yu et al. 2017).
Juglone growth response assays
To assess the sensitivity of candidate BCAs to juglone, we performed in vitro assays using half-strength PDA amended with five different concentrations of juglone (0.0 mg ml−1, 0.3 mg ml−1, 0.6 mg ml−1, 0.9 mg ml−1, and 1.2 mg ml−1) based on Wianowska et al. (2016). Juglone solutions were prepared by dissolving juglone (CAS 481-39-0; MiliporeSima™ Calbiochem™, St. Louis, MO, USA) in acetone (CAS 67-64-1; Fisher Chemical). Half-strength PDA was amended with juglone by spreading 350 μl of juglone solution onto the agar surface evenly with a sterile cell spreader and allowing the acetone to evaporate from the agar surface (Wianowska et al. 2016). Acetone alone (0 mg ml−1 juglone) was added to half-strength PDA as a control. Mycelial plugs (5 mm diameter) were taken from the growing margins of five to six-days old (Trichoderma) or seven-days-old cultures (B. bassiana strain GHA) and placed in the center of the amended Petri dishes. A total of ten replicates per juglone concentration were used for each isolate. Plates were incubated at 26 °C in the dark. Due to the rapid growth of Trichoderma spp. and B. bassiana, colonies were photographed two to three days after inoculation.
Trichoderma and Geosmithia morbida dual-plate antagonism assays
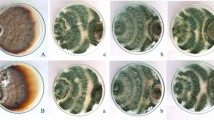
To assess the in vitro antagonistic activity of the Trichoderma isolates towards G. morbida, we performed two trials of dual-plate assays following the Gazis et al. (2018). In the first trial, all 15 Trichoderma isolates were characterized against G. morbida isolate TN Gm17 (Table 1). Prior to the addition of Trichoderma isolates, 5 mm mycelial plugs taken from the edges of six weeks old G. morbida colonies were placed on one side of a Petri dish containing half-strength PDA. Due to the comparatively slower growth rates of G. morbida isolates, we allowed G. morbida to grow prior to addition of rapid growing Trichoderma isolates (Gazis et al. 2018). When G. morbida colonies covered one-third of the agar surface (typically two to six weeks), Trichoderma plugs were placed on the opposing side of each Petri dish. Trichoderma plugs were harvested from the colony margins of seven-day-old cultures. A total of four replicates were used for each isolate (N = 60). For the second trial of dual-plate assays, we selected six Trichoderma isolates from the first trial (T. afroharzianum strain KRL-AG2, TN1-66, TN3-21, TN3-61, TN4-40, and TN4-47) and three G. morbida (CA Gm17-1, OR Gm62-3, and TN Gm17) isolates (Table 1) based on low sensitivity to juglone and antagonism toward G. morbida in the first trial of dual-plate assays. The second trial was conducted as described above. Negative controls consisted of half-strength PDA plates with a single 5 mm plug of each G. morbida isolate. For each G. morbida × Trichoderma isolate pairing in the second trial, we had five replicates (N = 90 plate pairs). In both trials, plates were incubated at 26 °C for 14 days in the dark and then scored on a scale of 1 to 4 (Fig. 2) (Gazis et al. 2018). Interactions were ranked 4 (no antagonistic activity) if no contact occurred between colonies, 3 if colonies came into contact but no overgrowth was observed, 2 if Trichoderma overgrew G. morbida but no sporulation was observed, and 1 (strong antagonistic activity) if Trichoderma overgrew and sporulated on G. morbida (Fig. 2).
Example of the scale used to rank Trichoderma interactions with Geosmithia morbida isolates. Interactions were ranked 4 if no contact occurred between colonies, 3 if colonies came into contact but no overgrowth was observed, 2 if Trichoderma overgrew G. morbida but no sporulation was observed, and 1 if Trichoderma overgrew and sporulated on G. morbida
Trichoderma antifungal metabolite assays
Isolates used in the second trial of the dual plate assays were screened for production of antifungal metabolites following the methodology described in Sundram (2013). Metabolite filtrates were prepared by first adding six 5 mm diameter plugs, collected from the margins of seven-day-old colonies of each Trichoderma isolate, to 1000 ml of half-strength potato dextrose broth. Broths were incubated at 26 °C and shaken at 150 rpm for 28 days. Fungal mycelia were separated from broths by vacuum filtration twice with a sterile 0.20 μm filter. To assess heat stability of filtrates, 400 ml of each filtrate was autoclaved at 121 °C for 15 min (Munimbazi and Bullerman 1998). We then amended half-strength PDA with filtrates at 30%, 50%, and 70% ratios. We used half-strength PDA with no metabolites added as a negative control (N = 45). A 5 mm diameter mycelial plug harvested from the growing margins of seven-day-old G. morbida colonies was placed into the center of each Petri dish. The study was designed as a 6 × 3 × 3 × 2 × 5 factorial (N = 540) with Trichoderma spp. (six isolates) × G. morbida (three isolates) × three concentrations of filtrate (30%, 50%, and 70%) × two heat treatments (autoclaved and not autoclaved) × five replicates. Petri dishes were incubated at 26 °C for seven days and were then photographed.
Image and statistical analysis
Colony area was measured using ImageJ v.1.53a (Schneider et al. 2012). Percent inhibition of colony area (Wianowska et al. 2016) was calculated and all statistical analyses were performed using R v.4.3.2 (https://github.com/aonufrak/trichoderma_antagonism_assays; R Core Team 2023). For all ANOVA tests described below we used the aov function from the stats package. We used Type III ANOVAs with the Anova function from the car package for all analyses to account for differences in sample size that resulted from mycelial plugs dislodging from the agar surface during the course of the experiments (Fox and Weisberg 2018). Significant pairwise group differences were determined using a Tukey’s post-hoc test (R Core Team 2023). To quantify the effects of juglone on the percent growth inhibition of B. bassiana and Trichoderma spp. isolates we used a two-way ANOVA with Trichoderma isolate identity included in the model to determine if there were differences among the growth responses of Trichoderma isolates to juglone. For antifungal metabolite assays, we used a three-way ANOVA to test for significant differences in the percent growth inhibition of G. morbida relative to the half-strength PDA control based on G. morbida isolate, Trichoderma metabolite broth, metabolite broth percentage, and their interaction for non-autoclaved and autoclaved heat treatments separately. We then tested for significant differences in the percent growth inhibition of G. morbida based on heat treatment, G. morbida isolate, the Trichoderma isolate that was used to make the filtrate, filtrate percentage, and interactions between factors.
Results
Juglone growth response assays
Mycelial plugs were dislodged from the surfaces of five acetone-only plates over the duration of the study and as a result, the final sample size was reduced from 50 to 45. Compared to the control treatment, B. bassiana strain GHA experienced a 50% growth reduction at 0.3 mg ml−1 and exceeded 75% at 1.2 mg ml−1 of juglone (F4, 40 = 59.7, P < 0.001; Fig. 3). In Trichoderma juglone assays, a total of 11 mycelial plugs dislodged from agar surfaces of juglone amended agar, resulting in a final sample size of 739. Responses of Trichoderma spp. to juglone were isolate-dependent (F56, 664 = 23.6, P < 0.001; Fig. 3). Isolates TN3-61 and TN4-47 did not experience a significant 50% reduction in growth below 1.2 mg ml−1 of juglone (Fig. 3). Growth inhibition of isolates TN3-21 and TN5-34 did not reach or exceed 50% under any tested concentration (Fig. 3). In juglone amended media, the remaining isolates, including T. afroharzianum strain KRL-AG2, had significant growth reductions (> 50%) at 0.6 mg ml−1 of juglone or greater (Fig. 3).
Percent inhibition of Beauveria bassiana strain GHA and fifteen Trichoderma isolates in response to five different concentrations of juglone. Letters above boxplots represent significant mean differences at a P < 0.05 determined using a Tukey’s post-hoc analysis. Dashed lines at 0% and 50% indicate points at which growth exceeded the mean of the negative no acetone control (0 mg ml−1) or growth was less than half the mean of negative no acetone control, respectively. Data for B. bassiana were analyzed separate from Trichoderma isolates. Negative values of percent inhibition represent instances in which colony growth exceeded the mean of the acetone control plates. The central line of each boxplot represents the median percent inhibition value, the upper and lower sections of each boxplot represent the 25th and 75th percentiles, and the upper and lower whiskers extend to the largest and smallest value that do not exceed 1.5 times the interquartile range for each treatment
Trichoderma and Geosmithia morbida dual-plate antagonism assays
In the first dual-plate assay trial, 12 of the 15 screened Trichoderma isolates (representing six different species) displayed strong antagonism (ranking of 1 or 2) towards G. morbida on half-strength PDA (Fig. 4a). Isolates KRL-AG2, NGM36-1, TN1-2, TN3-61, TN4-47, and TN5-34 sporulated through G. morbida (rank 1) in at least 50% of replicates (Fig. 4a). The remaining isolates were weak antagonists (rankings of 3 or 4) towards G. morbida (Fig. 4a). In the second trial of dual-plate assays, all isolates, except T. afroharzianum strain KRL-AG2 were strong antagonists (ranking of 1 or 2) towards three G. morbida isolates across all replicates (Fig. 4b). The commercial isolate T. afroharzianum strain KRL-AG2 had variable performance and was ranked as a weak antagonist (ranking of 3 or 4) of TN Gm17 in only two of five cases (Fig. 4b).
Trichoderma antifungal metabolite assays
Across the antifungal metabolite assays, 14 mycelial plugs became dislodged from agar surfaces resulting in a final sample size of 571 (autoclaved: N = 266; non-autoclaved: N = 260; control: N = 45). For both non-autoclaved and autoclaved metabolite filtrates, G. morbida experienced significant growth reductions relative to the control treatment based on the interactions between filtrate percentage and Trichoderma isolate (autoclaved: F12, 248 = 2.1, P = 0.01, non-autoclaved: F12, 242 = 1.8, P = 0.05; Fig. 5) as well as Trichoderma isolate and G. morbida isolate (autoclaved: F12, 248 = 2.0, P = 0.02, non-autoclaved: F12, 242 = 1.8, P = 0.05; Fig. 5). Heat treatment did not significantly affect the inhibitory activity of the filtrates at any percentage (F10, 418 = 1.7, P = 0.07). Filtrates made from T. afroharzianum strain KRL-AG2, TN1-66, and TN4-47 isolates significantly reduced the growth of G. morbida in relation to the half-strength PDA control at all metabolite broth percentages (Fig. 5). Filtrates made from isolate TN4-47 caused the greatest reduction in G. morbida growth (nearly 100% inhibition) compared to all other Trichoderma isolates regardless of amendment percentage or heat treatment (Fig. 5). The response of G. morbida to the remaining Trichoderma filtrates varied by Trichoderma isolate and amendment percentage (F10, 418 = 2.4, P = 0.009). Isolates TN3-21, T. afroharzianum strain KRL-AG2, TN4-40, TN3-61, and TN1-66 had significant increases in percent inhibition with increased filtrate concentration. However, of these five isolates, increasing the filtrate amendment to 70% resulted in a percent inhibition greater than 50% only for T. afroharzianum strain KRL-AG2 and TN1-66.
Percent growth inhibition of Geosmithia morbida isolates CA Gm17-1, OR Gm62-3, and TN Gm17 in response to non-heat treated (non-autoclaved) and heat treated (autoclaved) filtrates made using Trichoderma isolates TN3-21, TN4-47, T. afroharzianum strain KRL-AG2, TN4-40, TN3-61, and TN1-66 at 30%, 50%, and 70% concentrations. Points are shaped by G. morbida isolate. Letters above boxes indicate significant mean differences at P < 0.05. Dashed lines at 0% and 50% indicate points at which growth exceeded the mean of the no filtrate control or growth was less than half the mean of the no filtrate control, respectively. Negative values of percent inhibition represent instances in which colony growth exceeded the mean of the half-strength potato dextrose agar (PDA) control plates. The central line of each boxplot represents the median percent inhibition value, the upper and lower sections of each boxplot represent the 25th and 75th percentiles, and the upper and lower whiskers extend to the largest and smallest value that do not exceed the 1.5 times the interquartile range for each treatment
Discussion
Juglone is an allelochemical produced by walnut trees that has antifungal activity and, as hypothesized, affected the growth of candidate BCAs in our study (Wianowska et al. 2016). However, we identified four Trichoderma isolates (TN3-21, TN3-61, TN4-47, and TN5-34) that demonstrated greater tolerance to juglone compared to the other candidate BCAs in the study. Endophytic fungi often possess mechanisms to detoxify secondary metabolites that are produced by their associated host plants (Saunders and Kohn 2009). As such, we anticipated that the majority of the endophytic Trichoderma isolates tested in this study would outperform the commercial isolate (i.e., T. afroharzianum KRL-AG2) because the commercial isolate would be less likely to have evolved specialized mechanisms to tolerate host-specific secondary metabolites. However, the majority of Trichoderma isolates had growth responses to juglone similar to the commercial isolate.
Fungal responses to plant host secondary metabolites could explain the limited field efficacy observed for B. bassiana strain GHA (Castrillo et al. 2017, Mayfield et al. 2019). Beauveria bassiana strain GHA was comparatively less tolerant to juglone than the majority of endophytic Trichoderma spp. and the commercial Trichoderma isolate used in our study. Thus, while highly efficacious in laboratory settings, a lack of compatibility with host chemistry could limit the use of non-host plant-adapted isolates in the field. Previous research has demonstrated that the tolerance of B. bassiana isolates to host secondary metabolites is variable and has effects on fungal virulence (Mann and Davis 2020). As such, additional screening of B. bassiana isolates recovered from natural settings could allow for the identification of isolate(s) that can tolerate host plant secondary chemistry and become established in the microbial communities of walnut trees.
While we demonstrated that juglone can reduce in vitro growth of the identified potential BCAs, we do not currently know how the concentrations used in our study compare to the natural concentrations of juglone present in eastern black walnut phloem tissues across a growing season. Furthermore, it is unknown how these candidate BCAs will respond to other secondary metabolites potentially present in stem tissues, such as flavonoids, which may act synergistically with juglone to further reduce fungal growth (Wianowska et al. 2016). Previous research found that juglone-containing secondary metabolite extracts from English walnut (J. regia) husks yielded greater inhibitory effects than juglone standards alone due to the synergistic interactions of phenolic compounds with juglone (Wianowska et al. 2016). As a result, future research should quantify the effects of eastern black walnut metabolite extracts on the growth of candidate BCAs. When designing these future studies, consideration should also be given to the source of the extract including the plant host cultivar and tissue type and timing of sample collection (i.e., early or late growing season) as these are all factors that influence the concentrations of host secondary metabolites (Borazjani et al. 1985; de Scisciolo et al. 1990; Wianowska et al. 2016).
Similar to Gazis et al. (2018), 12 of the 15 examined Trichoderma isolates were consistently antagonistic towards G. morbida in dual plate assays. Biological control agents including fungal endophytes are often antagonistic towards phytopathogens in vitro (Halecker et al. 2020; Martínez-Arias et al. 2021). However, microbial interactions are context-dependent and can be impacted by a variety of abiotic and biotic factors (Mann and Davis 2020). Consideration of environmental factors is particularly important when developing biological management strategies for G. morbida, a xerotolerant fungus that can outcompete other fungi in arid conditions (Williams and Ginzel 2021). Thus, during periods of drought or in in more arid climates, the capability of these candidate BCAs may be affected. When designing field trials to examine the in planta efficacy of candidate BCAs, consideration should be given to how environmental factors such as humidity and temperature influence treatment efficacy (Mann and Davis 2020; Williams and Ginzel 2021).
In addition to being antagonistic towards G. morbida in our dual-plate assays, we identified a single Trichoderma isolate (TN4-47) that produced metabolites that nearly eliminated the growth of G. morbida. Fungal endophytes produce secondary metabolites that alter fungal community structure and modulate the establishment success of fungal pathogens (Rangel et al. 2021). Additionally, secondary metabolites produced by Trichoderma spp. can inhibit the growth of common phytopathogens (Vinale et al. 2006; Khare et al. 2018). Due to the relatively low sensitivity of Trichoderma isolate TN4-47 to juglone and the high efficacy and heat stability of the metabolite filtrate it produces, future research should identify the specific compound(s) responsible for the antifungal activity observed with this isolate, determine whether the compound is fungistatic or fungicidal, and characterize the impact of environmental factors (e.g., light, temperature, substrate) and chemical signals from cohabitating microorganisms including bacteria and fungi on metabolite production by isolate TN4-47 (Vinale et al. 2009; Keller 2019). Additional research could also consider the non-target effects of Trichoderma secondary metabolites when applied directly to black walnut for management of TCD (Keswani et al. 2014). Secondary metabolites produced by Trichoderma species not only directly affect target fungal pathogens but may confer additional benefits to host plants including increased plant growth and stimulation of host defenses (Keswani et al. 2014; Simamora et al. 2021).
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Bodenhofer U, Bonatesta E, Horejš-Kainrath C, Hochreiter S (2015) msa: an R package for multiple sequence alignment. Bioinformatics 31(24):3997–3999
Borazjani A, Graves C, Hedin P (1985) Occurrence of juglone in various tissues of pecan and related species. Phytopathol 75(12):1419–1421
Cai F, Druzhinina IS (2021) In honor of John Bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Divers 107:1–69
Castrillo LA, Mayfield AE III, Griggs MH, Camp R, Mudder B, Taylor A, Vandenberg JD (2017) Mortality and reduced brood production in walnut twig beetles, Pityophthorus juglandis (Coleoptera: Curculionidae), following exposure to commercial strains of entomopathogenic fungi Beauveria bassiana and Metarhizium brunneum. Biol Control 114:79–86
Chahal K, Gazis R, Klingeman W, Lambdin P, Grant J, Windham R, Hadziabdic D (2022) Differential virulence among Geosmithia morbida isolates collected across the United States occurrence range of thousand cankers disease. Front for Glob Change 5:726388
de Scisciolo B, Leopold DJ, Walton DC (1990) Seasonal patterns of juglone in soil beneath Juglans nigra (black walnut) and influence of J. nigra on understory vegetation. J Chem Ecol 16:1111–1130
Dou K, Lu Z, Wu Q, Ni M, Yu C, Wang M, Li Y, Wang X, Xie H, Chen J (2020) MIST: a multilocus identification system for Trichoderma. Appl Ecol Envrion Sci 86(18):e01532-e11520
Ferreira FV, Musumeci MA (2021) Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J Microbiol Biotechnol 37(5):90
Fox J, Weisberg S (2018) An R companion to applied regression, 3rd edn. Sage publications, Thousand Oaks
Gazis R, Poplawski L, Klingeman W, Boggess SL, Trigiano RN, Graves AD, Seybold SJ, Hadziabdic D (2018) Mycobiota associated with insect galleries in walnut with thousand cankers disease reveals a potential natural enemy against Geosmithia morbida. Fungal Biol 122(4):241–253
Hadziabdic D, Vito LM, Windham MT, Pscheidt JW, Trigiano RN, Kolarik M (2014) Genetic differentiation and spatial structure of Geosmithia morbida, the causal agent of thousand cankers disease in black walnut (Juglans nigra). Curr Genet 60:75–87
Halecker S, Wennrich J-P, Rodrigo S, Andrée N, Rabsch L, Baschien C, Steinert M, Stadler M, Surup F, Schulz B (2020) Fungal endophytes for biocontrol of ash dieback: the antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol 45:100918
Keller NP (2019) Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol 17(3):167–180
Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB (2014) Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl Microbiol Biotechnol 98:533–544
Khare E, Kumar S, Kim K (2018) Role of peptaibols and lytic enzymes of Trichoderma cerinum Gur1 in biocontrol of Fusraium oxysporum and chickpea wilt. Environ Sustain 1:39–47
Kolařík M, Freeland E, Utley C, Tisserat N (2011) Geosmithia morbida sp. nov., a new phytopathogenic species living in symbiosis with the walnut twig beetle (Pityophthorus juglandis) on Juglans in USA. Mycologia 103(2):325–332
Mann AJ, Davis TS (2020) Plant secondary metabolites and low temperature are the major limiting factors for Beauveria bassiana (Bals.-Criv.) Vuill.(Ascomycota: Hypocreales) growth and virulence in a bark beetle system. Biol Control 141:104130
Martínez-Arias C, Sobrino-Plata J, Ormeño-Moncalvillo S, Gil L, Rodríguez-Calcerrada J, Martín J (2021) Endophyte inoculation enhances Ulmus minor resistance to Dutch elm disease. Fungal Ecol 50:101024
Mayfield AE III, Juzwik J, Scholer J, Vandenberg JD, Taylor A (2019) Effect of bark application with Beauveria bassiana and permethrin insecticide on the walnut twig beetle (Coleoptera: Curculionidae) in black walnut bolts. J Econ Entomol 112(5):2493–2496
Munimbazi C, Bullerman LB (1998) Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. J Appl Microbiol 84(6):959–968
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. PNAS 95(5):2044–2049
Okonechnikov K, Golosova O, Fursov M, UGENE team (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28(8):1166–1167
Prospero S, Botella L, Santini A, Robin C (2021) Biological control of emerging forest diseases: how can we move from dreams to reality? For Ecol Manag 496:119377
R Core Team (2023) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.r-project.org
Rabiey M, Hailey LE, Roy SR, Grenz K, Al-Zadjali MA, Barrett GA, Jackson RW (2019) Endophytes vs tree pathogens and pests: can they be used as biological control agents to improve tree health? Eur J Plant Pathol 155:711–729
Rangel LI, Hamilton O, de Jonge R, Bolton MD (2021) Fungal social influencers: secondary metabolites as a platform for shaping the plant-associated community. Plant J 108(3):632–645
Saunders M, Kohn LM (2009) Evidence for alteration of fungal endophyte community assembly by host defense compounds. New Phytol 182(1):229–238
Schliep KP (2011) phangorn: phylogenetic analysis in R. Bioinformatics 27(4):592–593
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9(7):671–675
Schulz AN, Lucardi RD, Marsico TD (2019) Successful invasions and failed biocontrol: the role of antagonistic species interactions. BioScience 69(9):711–724
Simamora M, Basyuni M, Lisnawita L (2021) Potency of secondary metabolites of Trichoderma asperellum and Pseudomonas fluorescens in the growth of cocoa plants affected by vascular streak dieback. Biodiversitas 22(5):2542–2547
Sundram S (2013) The effects of Trichoderma in surface mulches supplemented with conidial drenches in the disease development of Ganoderma basal stem rot in oil palm. J Oil Palm Res 25(3):314–325
Tisserat N, Cranshaw W, Leatherman D, Utley C, Alexander K (2009) Black walnut mortality in Colorado caused by the walnut twig beetle and thousand cankers disease. Plant Health Prog 10(1):10
Vinale F, Marra R, Scala F, Ghisalberti E, Lorito M, Sivasithamparam K (2006) Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett Appl Microbiol 43(2):143–148
Vinale F, Ghisalberti E, Sivasithamparam K, Marra R, Ritieni A, Ferracane R, Woo S, Lorito M (2009) Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett Appl Microbiol 48(6):705–711
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, pp 315–322
Wianowska D, Garbaczewska S, Cieniecka-Roslonkiewicz A, Dawidowicz A, Jankowska A (2016) Comparison of antifungal activity of extracts from different Juglans regia cultivars and juglone. Microb Pathog 100:263–267
Williams GM, Ginzel MD (2021) Competitive advantage of Geosmithia morbida in low-moisture wood may explain historical outbreaks of thousand cankers disease and predict the future fate of Juglans nigra within its native range. Front for Glob Change 4:725066
Yu G, Smith DK, Zhu H, Guan Y, Lam TTY (2017) ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8(1):28–36
Acknowledgements
We would like to thank Beant Kapoor and Sarah Boggess for help with the maintenance and sub-culturing of fungal materials used in this study.
Funding
This work was supported by the USDA Forest Service Grant (19-DG11083150-010). Partial funding and support for students efforts were provided by the University of Tennessee (Department of Entomology and Plant Pathology, Herbert College of Agriculture, and Office of Undergraduate Research & Fellowships), USDA National Institute of Food and Agriculture AFRI Pre-Doctoral Fellowship (2022-67011-36578), and USDA National Institute of Food and Agriculture Hatch Projects #7002511 and #7004409 (IPM and Sustainable Strategies for Arthropod Pests and Plant Diseases in Nurseries, Managed Landscapes and Urban Forests and Forest Health and Resilience, respectively). Aaron Onufrak is currently funded by the Genomic Science Program of the US Department of Energy, Office of Science, Office of Biological and Environmental Research (BER) as part of the Secure Ecosystems Engineering and Design research program in the Secure Biosystems Design Scientific Focus Area (SFA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
No human participants or animals were included in the study.
Additional information
Handling Editor: James T. Tambong.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onufrak, A.J., Gazis, R., Gwinn, K. et al. Potential biological control agents of Geosmithia morbida restrict fungal pathogen growth via mycoparasitism and antibiosis. BioControl (2024). https://doi.org/10.1007/s10526-024-10277-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10526-024-10277-y