Abstract
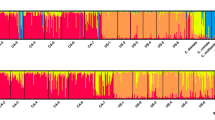
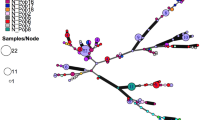
The main objectives of this study were to evaluate genetic composition of Geosmithia morbida populations in the native range of black walnut and provide a better understanding regarding demography of the pathogen. The fungus G. morbida, and the walnut twig beetle, Pityophthorus juglandis, have been associated with a disease complex of black walnut (Juglans nigra) known as thousand cankers disease (TCD). The disease is manifested as branch dieback and canopy loss, eventually resulting in tree death. In 2010, the disease was detected in black walnut in Tennessee, and subsequently in Virginia and Pennsylvania in 2011 and North Carolina in 2012. These were the first incidences of TCD east of Colorado, where the disease has been established for more than a decade on indigenous walnut species. A genetic diversity and population structure study of 62 G. morbida isolates from Tennessee, Pennsylvania, North Carolina and Oregon was completed using 15 polymorphic microsatellite loci. The results revealed high haploid genetic diversity among seven G. morbida populations with evidence of gene flow, and significant differentiation among two identified genetic clusters. There was a significant correlation between geographic and genetic distance. Understanding the genetic composition and demography of G. morbida can provide valuable insight into recognizing factors affecting the persistence and spread of an invasive pathogen, disease progression, and future infestation predictions. Overall, these data support the hypotheses of two separate, highly diverse pathogen introductions into the native range of black walnut.




Similar content being viewed by others
References
Amos W, Hoffman JI, Frodsham A, Zhang L, Best S, Hill VS (2007) Automated binning of microsatellite alleles: problems and solutions. Mol Ecol Notes 7:10–14
Becker DA, Brittingham MC, Goguen CB (2008) Effects of hemlock woolly adelgid on breeding birds at Fort Indiantown Gap, Pennsylvania. Northeast Nat 15:227–240
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Cranshaw W, Tisserat N (2008) Pest alert: walnut twig beetle and thousand cankers disease of black walnut. Colorado State University. http://wci.colostate.edu/Assets/pdf/ThousandCankers.pdf
Elliott KJ, Swank WT (2008) Long-term changes in forest composition and diversity following early logging (1919–1923) and the decline of American chestnut (Castanea dentata). Plant Ecol 197:155–172
Eschtruth AK, Battles JJ (2008) Deer herbivory alerts forest response to canopy decline caused by an exotic insect pest. Ecol Appl 18:360–376
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Grant JF, Windham MT, Haun WG, Wiggins GJ, Lambdin PL (2011) Initial assessment of thousand cankers disease on black walnut, Juglans nigra, in eastern Tennessee. Forests 2:741–748. doi:10.3390/f2030741
Hadziabdic D, Wadl PA, Vito LM, Boggess SL, Scheffler BE, Windham MT, Trigiano RN (2012) Development and characterization of sixteen microsatellite loci for Geosmithia morbida, the causal agent of thousand canker disease in black walnut (Juglans nigra). Conserv Genet Resour 4:287–289. doi:10.1007/s12686-011-9526-0
Hadziabdic D, Windham M, Baird E, Vito L, Cheng K, Grant J, Lambdin P, Wiggins G, Windham A, Merten P, Taylor G (2013) First report of Geosmithia morbida in North Carolina: the pathogen involved in thousand cankers disease of black walnut. Plant Dis. doi:10.1094/PDIS-06-13-0630-PDN
Hall B, Motzkin G, Foster DR, Syfert M, Burk J (2002) Three hundred years of forest and land-use change in Massachusetts, USA. J Biogeogr 29:1319–1335
Hansen MA, Bush E, Day E, Griffin G, Dart N (2011) Walnut thousand cankers disease alert, Virginia Tech-Virginia Cooperative Extension. http://www.vdacs.virginia.gov/plant&pest/disease-tcd.shtml
Hubisz M, Falush D, Stephens M, Pritchard J (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Res 9:1322–1332
Kolarik M, Kubatova A, Hulcr J, Pazoutova S (2008) Geosmithia fungi are highly diverse and consistent bark beetle associates: evidence from their community structure in temperate Europe. Microb Ecol 55:65–80
Kolarik M, Freeland E, Utley C, Tisserat N (2011) Geosmithia morbida sp. nov., a new phytopathogenic species living in symbiosis with the walnut twig beetle (Pityophthorus juglandis) on Juglans in USA. Mycologia 103:325–332
Langella O (1999) Populations 1.2.31. On web at: http://www.bioinformatics.org/~tryphon/populations/
Maliyakal EJ (1992) An efficient method for isolation of RNA and DNA from plants containing polyphenolics. Nucleic Acids Res 20:2381
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential and durable resistance. Annu Rev Phytopathol 40:349–379. doi:10.1146/annurev.phyto.40.120501.101443
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Nunes CF, Ferreira JL, Fernandes MCN, Breves SS, Generoso AL, Soares BDF, Dias MSC, Pasqual M, Borem A, Cançado GMA (2011) An improved method for genomic DNA extraction from strawberry leaves. Cienc Rural 41(8):1383–1389
Ordoñez ME, Kolmer JA (2007) Simple sequence repeat diversity of a worldwide collection of Puccinia triticina from durum wheat. Phytopathology 97:574–583
Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358. doi:10.1093/bioinformatics/12.4.357
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetics software for teaching and research—an update. Bioinformatics. First published online July 20, 2012. doi:10.1093/bioinformatics/bts460
Penn State-Penn State Cooperative Extension (2011) http://extension.psu.edu/greenindustry/news/2011/housand-cankers-disease-tcd-detected-in-pa
Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Pritchard JK, Donnelly P (2001) Case–control studies of association in structured or admixed populations. Theor Popul Biol 60:227–237
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Seybold SJ, Coleman TW, Dallara PL, Dart NL, Graves AD, Pederson LA, Spichiger SE (2012) Recent collecting reveals new state records and geographic extremes in the distribution of the walnut twig beetle, Pityophthorus juglandis Blackman (Coleoptera: Scolytidae), in the United States. Pan-Pacific Entomologist 88:277–280
Smouse PE, Long JC, Sokal RR (1986) Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst Zool 35(4):627–632
Stefansson TS, Serenius M, Hallsson JH (2012) The genetic diversity of Icelandic populations of two barley leaf pathogens, Rhynchosporium commune and Pyrenophora teres. Eur J Plant Pathol 134:167–180. doi:10.1007/s10658-012-9974-8
Tisserat N, Cranshaw W, Leatherman D, Utley C, Alexander K (2009) Black walnut mortality in Colorado caused by the walnut twig beetle and thousand cankers disease. Plant Health Prog. doi:10.1094/PHP-2009-0811-01-RS
Tsui CK, Roe AD, El-Kassaby YA, Rice AV, Alamouti SM, Sperling FA, Cooke JE, Bohlmann J, Hamelin RC (2012) Population structure and migration pattern of a conifer pathogen, Grosmannia clavigera, as influenced by its symbiont, the mountain pine beetle. Mol Ecol 21:71–86
USDA-APHIS (2009) Pathway assessment: Geosmithia sp. and Pityophthorus juglandis Blackman movement from the western into the eastern United States. http://www.tn.gov/agriculture/publications/regulatory/tc_pathwayanalysis.pdf
USDA-Forest Service (FS) and Plant Protection and Quarantine (PPQ) (2012) Thousand cankers disease Survey Guidelines for 2012. http://www.aphis.usda.gov/plant_health/plant_pest_info/tcd/downloads/TCDSurveyGuidelines2012.pdf
Utley C, Nguyen T, Roubtsova T, Coggeshall M, Ford TM, Grauke LJ, Graves AD, Leslie CA, McKenna J, Woeste K, Yaghmour MA, Cranshaw W, Seybold SJ, Bostock RM, Tisserat N (2013) Susceptibility of walnut and hickory species to Geosmithia morbida. Plant Dis 97:601–607
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis DGM, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wood SL, Bright DE (1992) A catalog of Scolytidae and Platypodidae (Coleoptera) 2. Taxonomic index. Great Basin Nat Mem 13:1–1533
Wright S (1969) Evolution nad the genetics of populations. Vol. II. The theory of gene frequencies. University of Chicago Press, Chicago
National Park Service, Great Smoky Mountains. http://www.nps.gov/grsm/parkmgmt/statistics.htm
Acknowledgments
The authors thank the United States Department of Agriculture-ARS (Grant number 58-6404-1-637), United States Forest Service (Grant number 13-DG-11083150-033) and United States Forest Service-Special Technology Development Program (Grant numbers 13-DG-11083150-039) for financial support. The authors thank independent reviewers for their comments and suggestions for manuscript improvement. Special thanks to undergraduate research assistants Dixie Daniels and Matthew Aldrovandi for their laboratory help, and Dana Rhodes (Department of Agriculture, PA), Richard Baird (Mississippi State University) and Qunkang Cheng (University of Tennessee) for collecting and obtaining additional samples for our study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Rights and permissions
About this article
Cite this article
Hadziabdic, D., Vito, L.M., Windham, M.T. et al. Genetic differentiation and spatial structure of Geosmithia morbida, the causal agent of thousand cankers disease in black walnut (Juglans nigra). Curr Genet 60, 75–87 (2014). https://doi.org/10.1007/s00294-013-0414-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-013-0414-x