Abstract
Assessment of acute toxicity of magnetic nanogel (MNG) is crucial to conclude the safe applicable dose and to warrant its application in aquaculture. Therefore, the current study is a novel step to assess behavior, neuro-stress response, hepato-renal, oxidative, and histopathological variations produced by MNG’ acute toxicity in Clarias gariepinus. Two experiments were conducted: the first was a determination of the 96-h lethal concentration 50 (LC50) of MNG in C. gariepinus. Meanwhile, the second was an assessment of the toxicological impacts of three different concentrations of MNG in C. gariepinus following a 10-day exposure period and a subsequent 10-day depuration trial. One hundred and eighty fish were allotted to four groups exposed to 0, 1/10, 1/8, or 1/5 96-h LC50 of MNG. The outcomes exhibited that 96-h LC50 of MNG for C. gariepinus was 44 mg/L. The subjected group to MNG induced a concentration-dependent elevation in the serum values of cortisol, alanine transaminase, aspartate transaminase, urea, and creatinine following MNG exposure. Marked elevation in the oxidative stress indicators (catalase (CAT), glutathione S-transferase activity (GST), and superoxide dismutase (SOD)) was also evident. Meanwhile, the value of the neurological biomarker, acetylcholinesterase (AChE), was markedly reduced in a concentration-dependent way. These biochemical changes were complemented by pathological alterations in the hepato-renal architecture. Interestingly, in response to the 10-day depuration period, most of the tested parameters were eliminated in C. gariepinus exposed to 1/10 of LC50. Conclusively, MNG can induce numerous adverse effects only at higher doses (1/5 and 1/8 of LC50). Meanwhile, the lowest tested concentration of MNG (1/10 of LC50) was safe for application in aquaculture practices with only mild disruptions in the bio-indices. In addition, a retrieval period of 10 days was sufficient to renovate these alterations only in fish exposed to the same concentration.
Similar content being viewed by others
Introduction
Utilization of nanomaterials originating from various materials has been used broadly in different applications (Neeti et al. 2023; Paul et al. 2023). Magnetite-based nanomaterials are one of the most successfully utilized nanoparticles which own magnetic characteristics (Kumari and Parashara 2018; Jv et al. 2019). The functionalized magnetic nanoparticles are beneficial in the treatment of wastewater because of their magnetic properties (Shahid et al. 2018). In spite of the reported safety of magnetite nanoparticles in aquaculture practices (Mahboub et al. 2021a), their toxicity has recently verified in zebrafish for inducing physiological and morphological alterations (Guillén et al. 2022). It could be returned to that the aquatic environment receives various pollutants which in turn enhances growth of nanomaterials (Halpern et al. 2015). Magnetite nanogel is another form of nanoparticle that is tiny and swollen and comprised of polymeric chains in nanosize (Duan et al. 2023). Currently, they are successfully practical in many applications due to their wide surface area and the power to load high percentage of water (Pinelli et al. 2023).
Nile catfish models are a fundamental principle of animal research in Egypt and have been extensively cultured and utilized to investigate the toxicity in the aquatic environment (Mahboub et al. 2021b; Abd El-Rahman et al. 2019).
Although the outcomes for using nanogels, either magnetite or titanium, are promising as an antioxidant agent and against bacterial infection (Rahman et al. 2023; Mahboub et al. 2024), their security in the aqueous environment must be assured prior their use. Therefore, the present work is a pioneer trial to investigate the acute toxicity exhibited by higher concentrations of MNG to assess the accurate safe dose for application. Further, we decided to investigate the efficacy of various concentrations of MNG on stress-neuro function, hepato-renal activity, hepatic antioxidant capacity, and histopathological alterations in Nile catfish.
Material and methods
Synthesis and characterization of MNG
At first, Fe3O4 NPs were synthesized as follows: an additional 0.4 g of the hematite ore (Fe3O4) was performed drop by drop to 40 mL of H2O2. Then, the mixture was exposed to ultrasound at 60 kHz for 2.5 h using an ultrasonic device (Sonica 4200 EPS3, Milano, Italy) till the formation of black particles of Fe3O4. After 1.5 h, the black-colored Fe3O4 NPs precipitated from the supernatant which is red in color. The separation of Fe3O4 NPs was carried out from the solution via centrifugation at 4000 rpm, and, lastly, the Fe3O4 NPs were rinsed four times using methanol. Characterization procedures were classified into three groups: morphology, identification, and index following the approach of Hassan et al. [15].
Fish and cultural condition
A total of 290 apparently healthy C. gariepinus (average body weight, 95.14 ± 0.45 g) were procured from a private fish farm in Sharkia province, Egypt. Prior to the initiation of the experiment, a 14-day acclimatization period was adopted for the fish to adapt to the laboratory conditions. During that duration, fish were reared in constantly aerated 120-L glass aquaria filled with dechlorinated tap water. The water in each aquarium was exchanged twice a week; meanwhile, the fish waste was siphoned daily. The water quality criteria were maintained within the optimum values along the course of the experiment, following Apha (1992): temperature, 25 ± 1.5 °C; dissolved oxygen, 6.55 ± 0.7 mg/L; pH, 6.70 ± 0.3; ammonia, 0.02 ± 0.001 mg/L; and nitrite, 0.015 ± 0.001 mg/L. The photoperiod was 12 h of light:12 h of darkness. During the adaptation period, fish received a basal diet (37% crud protein), formulated following the earlier protocol of Council (2011). To fulfill the nutritional requirements of C. gariepinus, they were fed at a rate of 3% of body weight, three times per day (8:00, 12:00, and 16:00 h).
Experimental design
Firstly, an acute experiment was conducted to monitor the 96-h LC50 of Magnetite nanogel (MNG) in C. gariepinus. For this purpose, 110 C. gariepinus were assigned into 11 groups each with 10 fish. The first group was maintained in clean dechlorinated water for 96 h and served as a control. The other ten groups were exposed to ten various levels of MNG (15, 20, 25, 30, 35, 40, 45, 50, 55, 60 mg/L). During the experimental period, neither the water was exchanged nor were the fish groups fed. The mortalities in all groups were recorded at 24, 48, 72, and 96 h. The behavioral, clinical symptoms, and post-mortem alterations were documented throughout the investigational period. The 96-h LC50 value was computed using Finney’s probit analysis (Finney 1971).
The second experiment (sub-acute exposure and recovery), included 180 C. gariepinus which were distributed into four equal groups, each group consisted of three replicates (15 fish/replicate, 45 fish/group). The first group was considered a control without any exposure. The other three groups were exposed to MNG at levels of 1/5, 1/8, and 1/10 of 96-h LC50, respectively. Fish were exposed to various concentrations of MNG for 10 days followed by a subsequent recovery period for another 10 days. Throughout this period, fish were kept in clean dechlorinated tap water.
Sampling
At the end of the two investigational periods, 15 fish samples were randomly selected from each group (5 fish per replicate) and anesthetized in ice-cold water. The blood was then collected from the caudal blood vessels using tubes without anticoagulants to isolate serum. Such serum was used for biochemical analysis including stress-related hormone, hepato-renal function, and the neurological indicator, AChE. In addition, the gills, liver, kidney, and intestine specimens were gathered and fixed for 48 h in 10% neutral buffered formalin for histopathological investigations. The liver homogenate was further processed to estimate oxidative stress indicators. Furthermore, spleen specimens were transported instantly in liquid nitrogen to be kept at −80 °C for the gene expression analysis of immune-related and apoptosis-related genes.
Hepatic oxidative stress indicators
Oxidative stress indices including catalase (CAT), glutathione S-transferase activity (GST), and superoxide dismutase (SOD) were assessed using colorimetric commercial kits (Biodiagnostic Co., Cairo, Egypt) following Aebi (1984), Habig et al. (1974), and Velkova-Jordanoska et al. (2008), respectively.
Serum biochemical parameters
The anti-stress indicator (cortisol level) was monitored using the spectrophotometry assay according to the previous method of Burtis and Ashwood (1994). The liver enzymes (alanine aminotransferase (ALT) and aspartate aminotransferases (AST)) and renal-damaged products (urea and creatinine) were computed in serum using the Spinreact kits (Esteve De Bas, Girona, Spain) following the protocol of Burtis and Ashwood (1994), Murray et al. (1984), Kaplan (1984), and Fossati et al. (1983), respectively. The activity of the neurological indicator, AChE, was spectrophotometrically determined depending on the assay of Ellman et al. (1961).
Histopathological investigation
Nine representative specimens from hepatic and renal tissues were freshly collected from the sacrificed C. gariepinus from each group. Preservation of these specimens was carried out by using 10% neutral-buffered formalin solution. Then, they were dehydrated via gradual emersion in ascending concentrations of ethanol, cleared in xylene, and fixed in paraffin. A microtome (Leica RM 2155, England) was used for sectioning of the samples to obtain a 5-micron thick sample, then stained with hematoxylin and eosin (Suvarna et al. 2018).
Statistical analysis
Firstly, the value of 96-h LC50 of MNG was calculated via using Finney’s probit analysis (Finney 1971). The data was confirmed for both normality and homogeneity of the variance by using by Levene’s tests. The outcomes of the impact of the grading concentrations of MNG during sub-acute exposure trial and after the depuration trial were scrutinized by using a two-way analysis of variance (two-way ANOVA) in SPSS version 18 (SPSS, Chicago, IL, USA). Tukey’s multiple range tests were used to evaluate the differences among means, and the results are displayed as means ± standard error (SE), and they were statistically significant at P < 0.05.
Results
Results of MNG characterization
Analysis of X-ray diffraction (XRD) revealed the fingerprint curve and data for magnetite based on the Brucker Database library, which aimed to confirm our synthesis protocol without any inferior phases. Zeta potential data and dynamic light scattering (DLS) revealed a regular size (one peak) of 60 nm (Fig. 1A). Because of a significant degree of zeta potential (−35 mV), the findings showed a greater colloidal structure in aqueous suspension (Fig. 1B).
Results of the acute toxicity experiment (MNG-96-h LC50 assays)
As illustrated in Fig. 2, fish exposed to different levels of MNG exhibited significant elevation in mortality rates in a concentration-reliant pattern, in comparison to the control group that recorded a 0% mortality rate. C. gariepinus exposed to the lower concentrations of MNG (15, 20, 25, 30, 35, and 40) for 96 h showed normal swimming movement and active response to escape reflex and other external stimuli. Nevertheless, fish exposed to higher concentrations of MNG for the same experimental period (96 h) were lethargic, anorexic, sluggish swimming movement, and weak response to the external stimuli. Moreover, hemorrhages and skin erosions were also evident. The mean 96-h LC50 MNG value for C. gariepinus was computed as 44 mg/L.
Results of sub-acute toxicity experiment and the recovery trial
Behavioral, clinical alterations, and post-mortem findings
Following the 10-day sub-acute exposure to different concentrations of MNG, the reaction to external stimuli was markedly and moderately decreased in fish subjected to 1/5 and 1/8 LC50 concentrations of MNG, respectively. However, C. gariepinus exposed to 1/10 LC50 MNG strongly reacted to external stimuli. Throughout the experimental duration, the exposed fish showed multiple clinical symptoms. Herein, the major detectable lesions were dark skin coloration, fin rot, and superficial hemorrhage at the base of the fins and ventral part of the abdomen (Table 1). The severity of the aforementioned signs was concentration related, where fish belonging to the 1/5 LC50 and 1/8 LC50 groups possessed the most announced signs, followed by those of the 1/10 LC50 group. Necropsy of freshly dead fish revealed congestion and enlargement of the liver and spleen, in addition to distention of the gall bladder.
Oxidative stress mediators
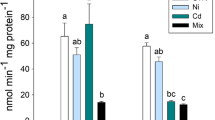
Concerning the effect of the two investigational trials on the serum cortisol level, the results indicated that after the sub-acute exposure experiment, C. gariepinus showed a significant enhancement in the activity of oxidative stress mediators (CAT, SOD, and GST) in comparison to their activity after the 10-day recovery trial (P < 0.01). Regarding the effect of the two investigational periods, the ascending increase in MNG concentration resulted in a remarkable elevation in the level of CAT, SOD, and GST, in a concentration-associated pattern (P < 0.01). The interaction between the effect of the investigational periods and MNG concentration yielded a significant increase in the serum levels of CAT, SOD, and GST. Such elevation was obvious especially following the sub-acute exposure to LC50, 1/8 LC50, followed by 1/10 LC50, then the control group. Interestingly, following the 10-day recovery trial, the 1/10 LC50 group’s level of these parameters displayed a remarkable improvement with a non-significant variation (P > 0.01) when compared with the control group. On the other hand, the hepatic levels of the antioxidant indicators revealed significantly boosted values in the 1/5 LC50 and 1/8 LC50 groups, in comparison to the 1/10 LC50 group and the control group (P < 0.01) (Table 2).
Stress-related corticosteroid hormone
The sub-acute exposure to MNG induced a marked rise in the cortisol level compared to its levels following the subsequent depuration experiment (P < 0.01). With regard to the effect of MNG concentration, the cortisol level was highly raised in a concentration-dependent manner (P < 0.01). The interaction between the two experimental periods and MNG concentration resulted in a significant reduction in the serum cortisol level in the 1/10 LC50 group compared with the 1/5 LC50 and 1/8 LC50 groups following the sub-acute exposure trial and the recovery trial (P < 0.01). Post the 10-day recovery period, the cortisol level was not significantly varied between the 1/10 LC50 group and the control one (P > 0.05) (Table 2). Additionally, the 1/5 LC50 and 1/8 LC50 groups maintained higher serum cortisol levels than the control group even after the 10-day recovery period.
Hepato-renal injury indices
The serum levels of ALT, AST, urea, and creatinine revealed a remarkable rise (P < 0.01), following the 10-day exposure trial compared to their levels after the subsequent recovery trial (Table 3). Regarding the investigational trial effect, a concentration-dependent elevation in the levels of the hepato-renal indices was detected concurrently with increasing concentration of MNG surpassing the control group (P < 0.01). The interaction between the investigational trial and MNG concentration showed that AST, ALT, urea, and creatinine levels were significantly increased in fish exposed to various concentrations of MNG respective to their values in the control group (P < 0.05). In response to the recovery trial, the serum values of AST, urea, and creatinine exhibited a non-significant difference (P > 0.05) between fish exposed to 1/10 LC50 and the control group. In contrast, the ALT level possessed a significant rise in this group relative to the control one (P < 0.05). Furthermore, the level of hepato-renal damage indices was still higher in the 1/5 and 1/8 LC50 groups than in the control group (P < 0.05) and the 1/10 LC50 group.
Acetylcholinesterase (AChE) level
Considering the MNG concentration, the serum values of the neurotransmitter, AChE, were markedly declined in all groups following the sub-acute exposure period compared to its level post-recovery (P < 0.01). With regard to the effect of the investigational trial, the level of AChE exhibited a significant concentration-related reduction in MNG-exposed fish compared to the control group (P < 0.01). Moreover, the interaction between the investigational trials and MNG concentration indicated that the sub-acute exposure of C. gariepinus to higher concentrations of MNG (1/5 and 1/8 LC50) resulted in a significant depletion in AChE activity compared to the control group. But, this effect was recorded in fish exposed to 1/10 LC50 to a lesser extent. Nevertheless, after the recovery trial, the serum values of AChE were not significantly varied between the 1/10 LC50 group and the control group (P > 0.05). The level of AChE was higher in both the 1/5 LC50 and 1/8 LC50 groups relative to the 1/10 LC50 and the control groups (P < 0.05) (Table 3).
Histopathological investigations
Considering the histopathological outcomes of the renal tissue, the control group showed normal morphology of glomerular corpuscles, renal tubules, and abundant interstitial hematopoietic series (Fig. 3A), while fish exposed to 1/5 96-h LC50 revealed necrosis with pyknotic nuclei in most renal tubular epithelium accompanied with atrophic glomerular tufts (Fig. 3B). Moreover, moderate number of necrotic renal tubules were seen in fish exposed to 1/8 96-h LC50 (Fig. 3C) with normal morphology of glomeruli. But, necrotic changes in mild number of renal tubules besides normal morphology of glomeruli were seen in exposed to 1/10 96-h LC50 (Fig. 3D). Ten days after cessation of exposure to MNG, the renal tissue of fish exposed to 1/5 96-h LC50 revealed degenerative and necrotic alterations in few renal tubular epitheliums with normal glomerular tufts (Fig. 3E). On the other hand, focal dissociation of renal epithelium in some renal tubules with preserved structures of glomerular tufts was observed in fish exposed to 1/8 96-h LC50 (Fig. 3F). Surprisingly, prominent improvement in architectures of glomeruli and renal tubules was observed in fish exposed to 1/10 96-h LC50 (Fig. 3G).
Representative photomicrograph of H&E stained sections from the kidneys (scale bar 20 μm) showing A normal morphology of glomerular corpuscle (arrow), renal tubule (arrowhead), and abundant of interstitial hematopoietic series in control group. B Necrosis with pyknotic nuclei in most renal tubular epithelium (arrowhead) accompanied with atrophic glomerular tufts (arrow) in C. gariepinus sub-acutely exposed to 1/5 LC50 MNG. C Moderate number of necrotic renal tubules (arrowhead) with normal morphology of glomeruli (arrow) in C. gariepinus sub-acutely exposed to 1/8 LC50 MNG. D Necrotic changes in mild number of renal tubules (arrowhead) beside normal morphology of glomeruli (arrow) in C. gariepinus sub-acutely exposed to 1/10 LC50 MNG. E Degenerative and necrotic changes in few renal tubular epitheliums (arrowhead) with normal glomerular tufts (arrow) in 1/5 LC50 group after recovery trial. F Focal dissociation of renal epithelium in some renal tubules (arrowhead) with preserved structures of glomerular tufts (arrow) in in 1/8 LC50 group after recovery trial. G Prominent improvement in architectures of glomeruli (arrow) and renal tubule (arrowhead) in 1/10 LC50 group after recovery trial
Regarding the histopathological findings of the hepatic tissue following the 10-day exposure period to MNG, the control group revealed normal hepatic acini, sinusoids, and central veins (Fig. 4A). As shown in Fig. 4B, fish exposed to 1/5 LC50 displayed a large number of vacuolated hepatocytes and congested vasculatures. Fish exposed to 1/8 LC50 revealed scattered round cell infiltrates and a moderate number of vacuolated hepatocytes (Fig. 4C), whereas mild degenerative changes in some hepatic cells were the most characteristic findings shown by those exposed to 1/10 LC50 (Fig. 4D). On the other hand, at the end of the 10-day recovery period, there was still the presence of degenerative changes in few hepatocytes beside minute numbers of round cells aggregates were noticed in fish previously exposed to 1/5 LC50 (Fig. 4E). Moreover, as displayed in Fig. 4F, fish exposed to 1/8 LC50 exhibited mild degenerative changes in few hepatocytes. However, a remarkable improvement in the architectures of hepatic acini and their vasculatures was evident in fish exposed to 1/8 LC50 (Fig. 4G).
Representative photomicrograph of H&E stained sections from the liver (scale bar 20 μm) showing A normal hepatic acini (arrow), sinusoids, and central vein (arrowhead) in control group. B Large number of vacuolated hepatocytes (arrowhead) and congested vasculatures (arrow) in C. gariepinus sub-acutely exposed to 1/5 LC50 MNG. C Scattered round cells infiltrates (arrow) and moderate number of vacuolated hepatocytes (arrowhead) in C. gariepinus sub-acutely exposed to 1/8 LC50 MNG. D Mild degenerative changes in some hepatic cells (arrow) in C. gariepinus sub-acutely exposed to 1/10 LC50 MNG. E Degenerative changes in few hepatocytes (arrowhead) and minute number of round cells aggregates (arrow) in 1/5 LC50 group after recovery trial. F Mild degenerative changes in few hepatocytes (arrow) in 1/8 LC50 group after recovery trial. G Improvement of architectures of hepatic acini (arrow) and vasculature tissue (arrowhead) in 1/10 LC50 group after recovery trial
Discussion
Nowadays, the field of nanotechnology has played a crucial role in aquaculture practices for effectively utilizing nanoparticles as a dietary supplement (Ahmed et al. 2023), in spite of its reported toxicity (Kakakhel et al. 2023). Exposure of fish to metal oxide nanoparticles can induce malformation of different organs, abnormal behavior, immune dysfunction, genotoxicity, and higher mortalities (Cazenave et al. 2019; Bai and Tang 2020). Therefore, the current report is pioneering approach for the assessment of acute toxicity elicited by MNG via testing various concentrations and studying its influence on the stress response, hepato-renal indices, antioxidant mechanism, and histopathological changes in hepato-renal organs.
Considering clinical signs and mortality records, the present study monitored the mortalities throughout the experimental period and revealed the occurrence of mortalities with increasing the concentration of MNG. In addition, fish demonstrated clinical manifestations including dark coloration of the skin, severe fin rot, and some hemorrhages in the skin. Internally, the examined fish revealed congestion and enlargement of the liver and distention of the gall bladder, especially at higher doses of MNG. The severity of mortalities and clinical signs was associated with augmenting the dose of MNG implying MNG toxicity at higher doses (1/5 and 1/8 of LC50), while the exposure to 1/10 LC50 demonstrated the lowest death rate and clinical signs. The signs of toxicity and severity of mortalities provoked by magnetite nanocomposite depend on the period of exposure and its concentration as reported by Zhu et al. (2012). Likewise, a recent study by Guillén et al. (2022) supported our findings and revealed that the exposure of zebrafish to a high concentration (1000 μg/mL) of magnetite-based nanocomposites induced morphological alterations. In line with an earlier study, Zhu et al. (2012) mentioned the occurrence of ulcerations, pericardial edema, and spinal curvature post-acute exposure of zebrafish embryos to magnetite nanocomposites for 168 h.
Assessment of oxidative stress is crucial to reflect the adverse effect of nanoparticles in catfish which resulted in a reduction of antioxidant enzymes including CAT, GST, and SOD (Iheanacho and Odo 2020; Iheanacho et al. 2021). Oxidative stress is raised because of an imbalance between the antioxidant resistance of the host and the release of ROS after exposure to stressors including nanomaterials (Song et al. 2023; Alzahrani et al. 2022; El-Houseiny et al. 2023). The enzymatic antioxidant defense mechanism is responsible for mitigating the production of ROS involving CAT, SOD, and GST enzymes and antagonizing the toxicity in C. gariepinus (El-Houseiny et al. 2023). To tackle the oxidative damage induced by MNG on the antioxidant defense system, the present perspective monitored different concentrations of acute exposure to MNG. Additionally, it determined their impact on the antioxidant enzymes (SOD, CAT, and GST) and established a fact of the existence of oxidative disruption indicated by an increase in the antioxidant biomarkers in a dose-based manner. Astonishingly, after a 10-day recovery period, the 1/10 LC50 group’s level of these indicators exhibited a clear improvement reflecting a potent antioxidant activity of MNG at lower concentration. It is opined that MNG has cytotoxic effects via the occurrence of inflammatory responses and oxidative damage or could be returned to the ability of magnetite nanocomposites to suppress the Na+/K+-ATPase activity in a concentration-reliant array as clarified by Suganya et al. (2018). Similar report by Kaloyianni et al. (2020) was conducted on zebrafish, Danio rerio, and revealed the occurrence of oxidative stress in the gills and liver indicated by elevation of lipid peroxidation and protein oxidation.
Valuation of acute toxicity of freshwater fish to various nanoparticles is crucial to evaluate the accurate safe dose for application, and in turn, avoid the occurrence of stress condition and hepato-renal dysfunction (Rashidian et al. 2021). Cortisol is a vital stress indicator in fish and is a precise marker for evaluation of acute stress (Sadoul and Geffroy 2019). Herein, we reveal elevation in cortisol, liver enzymes (ALT, AST), and kidney biomarkers (urea, creatinine). Such elevations were concurrent with the increased concentration of MNG representing a persuasive stress response and hepato-renal dysfunction. Nonetheless, the serum values of AST, urea, and creatinine exhibited a non-significant difference (P > 0.05) in the exposed group to the 1/10 LC50 group and the control implying the MNG safety at a lower dose. These findings were synchronized with a recent study by Rahman et al. (2022), who reported the existence of stress status symbolized by an elevation in the cortisol upon exposure to the acute toxicity by silica nanoparticles in C. gariepinus.
Acetylcholinesterase (AChE) has an essential role in the nervous system representing in breaking down acetylcholine into acetic acid and choline (Ibrahim et al. 2022; Kim and Kang 2015). Consequently, AChE conserves correct levels of acetylcholine and inhibits AChE resulting in accretion of acetylcholine at the synaptic junctions, indicating neurotoxicity (Kais et al. 2015). In the present investigation, it was perceived that the exposure of C. gariepinus to MNG lessened the brain biomarkers (AChE) in a dose-dependent pattern, implying neurological dysfunction and oxidative damage. On the other hand, an obvious restoration of the level of this neurotransmitter activity was evident after the 10-day depuration period, especially at a lower dose of exposure to MNG (1/10 LC50). It is assumed that the MNG can translocate straight away from the olfactory nerve to reach the brain inducing neurotoxic impacts as previously described by Wu et al. (2013). Likewise, Yousef et al. (2019) revealed that the exposure to magnetite nanocomposite resulted in neurotoxicity and inflammatory response in rats, indicated by a reduction in the AChE.
Regarding the ecotoxicological effect of MNG on the architecture of hepato-renal organs, we reported the existence of pathological changes in the liver and kidneys especially in the exposed groups to higher concentrations of MNG (1/5 and 1/8 of LC50). Surprisingly, a noticeable regeneration in the renal architectures was observed in the fish exposed to 1/10 96-h LC50 implying the safety of MNG when applied at low concentration. Concurrent with previous reports, Qualhato et al. (2018) recorded the occurrence of hepatic alterations in guppy (Poecilia reticulata) throughout 3 weeks of exposure to magnetite nanosized particles. Moreover, Rezende et al. (2018) noted histopathological alterations in Oreochromis niloticus upon exposure to titanium dioxide nanoparticles.
Conclusion
Overall, the sub-acute toxicity with MNG induces adverse impacts, only at higher doses, 1/5 LC50 and 1/8 LC50. The ethological alteration, hepato-renal dysfunction, oxidative stress, neurological disorders, and histopathological changes represented the major negative impacts provoked by MNG toxicity in C. gariepinus. Nevertheless, the lowest applied concentration of MNG, 1/10 of LC50, was less toxic, so it could be safely applied in aquaculture practices with only minor disruptions in the bio-indicators. Future studies are required to test the influence of MNG on the immune system and gene expression.
Data availability
The datasets generated or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Aebi H (1984) Catalase in vitro. In: Methods in enzymology, vol 105. Elsevier, pp 121–126
Abd El-Rahman GI, Ahmed SAA, Khalil AA, Abd-Elhakim YM (2019) (2019) Assessment of hematological, hepato-renal, antioxidant, and hormonal responses of Clarias gariepinus exposed to sub-lethal concentrations of oxyfluorfen. Aquat Toxicol 217:105329
Ahmed SA, El-Murr A, Abd Elhakim Y, Metwally MM, Gharib AAEA, Amer SA, Younis EM, Abdel-Warith A-WA, Davies SJ, Khalil EN (2023) Comparative study on ginger powder and ginger extract nanoparticles: effects on growth, immune–antioxidant status, tissue histoarchitecture, and resistance to Aeromonas hydrophila and Pseudomonas putida infection in Oreochromis niloticus. Fishes 8(5):259
Alzahrani OM, Elumalai P, Nada HS, Ahmed SA, Zaglool AW, Shawky SM, Alkafafy M, Mahboub HH (2022) Pseudomonas putida: sensitivity to various antibiotics, genetic diversity, virulence, and role of formic acid to modulate the immune-antioxidant status of the challenged nile tilapia compared to carvacrol oil. Fishes 8(1):6
Apha A (1992) WPCF (American Public Health Association, American Waterworks Association, Water Pollution Control Federation)(1992) standard methods for the examination of water and wastewater. Stand Methods Exam Water Wastewater 17
Bai C, Tang M (2020) Toxicological study of metal and metal oxide nanoparticles in zebrafish. J Appl Toxicol 40(1):37–63
Burtis CA, Ashwood ER (1994) Tietz textbook of clinical chemistry. Amer Assn for Clinical Chemistry
Cazenave J, Ale A, Bacchetta C, Rossi AS (2019) Nanoparticles toxicity in fish models. Curr Pharm Des 25(37):3927–3942
Duan Q-Y, Zhu Y-X, Jia H-R, Wang S-H, Wu F-G (2023) Nanogels: synthesis, properties, and recent biomedical applications. Prog Mater Sci 139:101167. https://doi.org/10.1016/j.pmatsci.2023.101167
El-Houseiny W, AbdelMageed M, Abd-Elhakim YM, Abdel-Warith A-WA, Younis EM, Abd-Allah NA, Davies SJ, El-Kholy MS, Ahmed SA (2023) The effect of dietary Crataegus sinaica on the growth performance, immune responses, hemato-biochemical and oxidative stress indices, tissues architecture, and resistance to Aeromonas sobria infection of acrylamide-exposed Clarias gariepinus. Aquaculture Reports 30:101576
Ellman GL, Courtney KD, Andres V, Featherstone RM. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7 (2):88-95. doi:https://doi.org/https://doi.org/10.1016/0006-2952(61)90145-9
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge, UK
Fossati P, Prencipe L, Berti G (1983) Enzymic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin Chem 29(8):1494–1496
Guillén A, Ardila Y, Noguera MJ, Campaña AL, Bejarano M, Akle V, Osma JF (2022) Toxicity of modified magnetite-based nanocomposites used for wastewater treatment and evaluated on zebrafish (danio rerio) model. Nanomaterials 12(3):489
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Halpern BS, Frazier M, Potapenko J, Casey KS, Koenig K, Longo C, Lowndes JS, Rockwood RC, Selig ER, Selkoe KA (2015) Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat Commun 6(1):1–7
Ibrahim RE, Ghamry HI, Althobaiti SA, Almalki DA, Shakweer MS, Hassan MA, Khamis T, Abdel-Ghany HM, Ahmed SA (2022) Moringa oleifera and Azadirachta indica Leaves enriched diets mitigate chronic oxyfluorfen toxicity induced immunosuppression through disruption of pro/anti-inflammatory gene pathways, alteration of antioxidant gene expression, and histopathological alteration in Oreochromis niloticus. Fishes 8(1):15
Iheanacho SC, Adeolu AI, Nwose R, Ekpenyong J, Offu P, Amadi-Eke A, Iheanacho AC, Ogunji J (2021) Genotoxicity, oxidative stress and lysozyme induction in Clarias gariepinus chronically exposed to water-soluble fraction of burnt tire ash. Ecotoxicology 30:1983–1996
Iheanacho SC, Odo GE (2020) Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Compara Biochem Physiol C: Toxicol Pharmacol 232:108741
Jv X, Zhao X, Ge H, Sun J, Li H, Wang Q, Lu H (2019) Fabrication of a magnetic poly (aspartic acid)-Poly (acrylic acid) hydrogel: application for the adsorptive removal of organic dyes from aqueous solution. J Chem Eng Data 64(3):1228–1236
Kais B, Stengel D, Batel A, Braunbeck T (2015) Acetylcholinesterase in zebrafish embryos as a tool to identify neurotoxic effects in sediments. Environ Sci Pollut Res 22:16329–16339
Kakakhel MA, Bibi N, Mahboub HH, Wu F, Sajjad W, Din SZU, Hefny AA, Wang W (2023) Influence of biosynthesized nanoparticles exposure on mortality, residual deposition, and intestinal bacterial dysbiosis in Cyprinus carpio. Compara Biochem Physiol C: Toxicol Pharmacol 263:109473
Kaloyianni M, Dimitriadi A, Ovezik M, Stamkopoulou D, Feidantsis K, Kastrinaki G, Gallios G, Tsiaoussis I, Koumoundouros G, Bobori D (2020) Magnetite nanoparticles effects on adverse responses of aquatic and terrestrial animal models. J Hazard Mater 383:121204
Kaplan A (1984) Urea. Kaplan A. Clin Chem The CV Mosby Co. St Louis. Toronto. Princeton 1984; 1257–1260 and 437 and. 418.
Kim J-H, Kang J-C (2015) Oxidative stress, neurotoxicity, and non-specific immune responses in juvenile red sea bream, Pagrus major, exposed to different waterborne selenium concentrations. Chemosphere 135:46–52
Kumari P, Parashara H (2018) β-cyclodextrin modified magnetite nanoparticles for efficient removal of eosin and phloxine dyes from aqueous solution. Mater Today: Proc 5(7):15473–15480
Mahboub HH, Eltanahy A, Omran A, Mansour AT, Safhi FA, Alwutayd KM, Khamis T, Husseiny WA, Ismail SH, Yousefi M (2024) Chitosan nanogel aqueous treatment improved blood biochemicals, antioxidant capacity, immune response, immune-related gene expression and infection resistance of Nile tilapia. Comp Biochem Physiol B: Biochem Mol Biol 269:110876
Mahboub HH, Beheiry RR, Shahin SE, Behairy A, Khedr MH, Ibrahim SM, Elshopakey GE, Daoush WM, Altohamy DE, Ismail TA (2021a) Adsorptivity of mercury on magnetite nano-particles and their influences on growth, economical, hemato-biochemical, histological parameters and bioaccumulation in Nile tilapia (Oreochromis niloticus). Aquat Toxicol 235:105828
Mahboub HH, Khedr MH, Elshopakey GE, Shakweer MS, Mohamed DI, Ismail TA, Ismail SH, Rahman ANA (2021b) Impact of silver nanoparticles exposure on neuro-behavior, hematology, and oxidative stress biomarkers of African catfish (Clarias gariepinus). Aquaculture 544:737082
Murray JF, Felton CP, Garay SM, Gottlieb MS, Hopewell PC, Stover DE, Teirstein AS (1984) Pulmonary complications of the acquired immunodeficiency syndrome: report of a National Heart, Lung, and Blood Institute workshop. N Engl J Med 310(25):1682–1688
Neeti K, Singh R, Ahmad S (2023) The role of green nanomaterials as effective adsorbents and applications in wastewater treatment. Mater Today: Proc 77:269–276
Paul S, Mukherjee S, Banerjee P (2023) Recent advancement in the nanomaterials encapsulated drug delivery vehicles towards combating of cancer, COVID-19 and HIV like chronic diseases. Mater Adv 4:2042–2061
Pinelli F, Saadati M, Zare EN, Makvandi P, Masi M, Sacchetti A, Rossi F (2023) A perspective on the applications of functionalized nanogels: promises and challenges. Int Mater Rev 68(1):1–25
Qualhato G, de Sabóia-Morais SMT, Silva LD, Rocha TL (2018) Melanomacrophage response and hepatic histopathologic biomarkers in the guppy Poecilia reticulata exposed to iron oxide (maghemite) nanoparticles. Aquat Toxicol 198:63–72
Rahman ANA, Elkhadrawy BA, Mansour AT, Abdel-Ghany HM, Yassin EMM, Elsayyad A, Alwutayd KM, Ismail SH, Mahboub HH (2023) Alleviating effect of a magnetite (Fe3O4) nanogel against waterborne-lead-induced physiological disturbances, histopathological changes, and lead bioaccumulation in African Catfish. Gels 9(8):641
Rahman ANA, Shakweer MS, Algharib SA, Abdelaty AI, Kamel S, Ismail TA, Daoush WM, Ismail SH, Mahboub HH (2022) Silica nanoparticles acute toxicity alters ethology, neuro-stress indices, and physiological status of African catfish (Clarias gariepinus). Aquaculture Reports 23:101034
Rashidian G, Lazado CC, Mahboub HH, Mohammadi-Aloucheh R, Prokić MD, Nada HS, Faggio C (2021) Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) skin mucus. Int J Mol Sci 22(6):3270
Rezende KFO, Bergami E, Alves KVB, Corsi I, Barbieri E (2018) Titanium dioxide nanoparticles alter routine metabolism and cause histopathological alterations in Oreochromis niloticus. Bol Inst Pesca 44(2)
Sadoul B, Geffroy B (2019) Measuring cortisol, the major stress hormone in fishes. J Fish Biol 94(4):540–555
Shahid MK, Phearom S, Choi Y-G (2018) Synthesis of magnetite from raw mill scale and its application for arsenate adsorption from contaminated water. Chemosphere 203:90–95
Song C, Sun C, Liu B, Xu P (2023) Oxidative stress in aquatic organisms, vol 12. MDPI
Suganya D, Ramakritinan C, Rajan M (2018) Adverse effects of genotoxicity, bioaccumulation and ionoregulatory modulation of two differently synthesized iron oxide nanoparticles on zebrafish (Danio rerio). J Inorg Organomet Polym Mater 28:2603–2611
Suvarna KS, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques. Elsevier health sciences
Velkova-Jordanoska L, Kostoski G, Jordanoska B (2008) Antioxidative enzymes in fish as biochemical indicators of aquatic pollution. Bulgarian J Agr Sci 14(2):235–237
Wu J, Ding T, Sun J (2013) Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology 34:243–253
Yousef MI, Abuzreda AA, Kamel MAE-N (2019) Neurotoxicity and inflammation induced by individual and combined exposure to iron oxide nanoparticles and silver nanoparticles. Journal of Taibah University for Science 13(1):570–578
Zhu X, Tian S, Cai Z. (2012) Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages.
Acknowledgements
This work was supported by the Researches Supporting Project number (RSP2024R36), King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was funded by Researches Supporting Project number (RSP2024R36), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization: SK, SAAA, AE, HHM, MS, EMY, AAA, MMSG, TK, SHI, SJD, and ZH; methodology: SK, SAAA, HHM, MS, EMY, AAA, MMSG, TK, SHI, SJD, and ZH; software and data curation: SK, SAAA, HHM, MS, EMY, AAA, MMSG, TK, SHI, SJD, and ZH; writing—original draft preparation: SAAA, SK, and HHM; writing—reviewing and editing: HHM and SAAA
Corresponding authors
Ethics declarations
Ethics approval
All experimental procedures with live fish were approved by the animal welfare and ethical review committee of Faculty of Veterinary Medicine, Zagazig University, Egypt (ZU-IACUC/2/F/435/2023). All experimental procedures were directed in compliance with the ethical guidelines agreed by the National Institutes of Health for Use and Treatment of Laboratory Animals.
Informed consent
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamel, S., Ahmed, S.A.A., Elsayyad, A. et al. Toxicological insight of magnetite nanogel: neuro-ethological, hepato-renal, antioxidant, and histopathological traits in Clarias gariepinus. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01456-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01456-w