Abstract
Fish transportation is a regular farming and experimental practice that mainly results in stress conditions that may extend to induce a high mortality rate. In the present experimental simulation, Oreochromis niloticus were transported for 2 h and maintained for another 6 h in transportation containers to assess the effect of tranquilization with tricaine methanesulfonate (MS-222) and/or the addition of iodine on mortality rate and restoring the normal physiological functions. Experimental fish were divided into four groups: group 1 (control) was transported in farm water without MS-222 or iodine, group (2) was transported in farm water supplemented with 40 mg/L of MS-222, fish in group (3) were transported in farm water supplemented with 40 mg/L of MS-222 + 10 ppm iodine, and fish in group (4) were transported in farm water containing 10 ppm iodine. Blood samples were collected for the determination of serum cortisol and glucose, while skin mucus was collected for assaying lysozyme, peroxidase and antibacterial activity; cumulative mortality rate; and food reflexes which were assessed at 0 h, 1 h, 6 h, 48 h, 7 days and 14 days post 2 h transportation. The results indicated that fish tranquilization with MS-222 and/or treatment with iodine mitigated the stress condition associated with transportation and accelerate restoration of the normal physiological and immunological status. So, it was recommended to use the MS-222 as a tranquillizer together with iodine as an antiseptic during transportation of O. niloticus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nile tilapia is a popular cultured fish, and is farmed in > 100 countries (FAO 2018). The success is attributed to easy culturing, rapid growth and high profitability (Prabu et al. 2019). The intensification of Nile tilapia farming has raised the stress levels resulting in high mortality rate and subsequent huge financial losses (Chen et al. 2019). Fish skin secretes mucus via goblet cells (Sanahuja et al. 2019) that exerts a vital role as a physical, biological and immunological barrier against external invaders through the non-specific immunity parameters present in mucus such as proteases, antiproteases, peroxidases, esterases, alkaline phosphatase, lysozyme and immunoglobulins (Brinchmann 2016; Carda-Diéguez et al. 2017).
Fish transportation during aquaculture routine work and for conducting scientific experiments on fish is a regular process that induces stress responses, and so, anaesthesia and tranquilization are necessary during this process (Simões et al. 2011; Sampaio and Freire 2016), to avoid negative impacts and stress (Park et al. 2008). However, the strength of stress responses is variable based on the transported fish species, size and stress duration (Sumpter et al. 1985).
Tricaine methanesulfonate (MS-222) has a low effective drug dose, fast anaesthesia, rapid recovery and no toxic side effects on treated fish (Popovic et al. 2012); it is also considered the only anaesthetic approved by the Food and Drug Administration (USFDA) for use in food fish (Park et al. 2017). The recommended concentration of MS-222 differs according to fish species; it is 25 to 100 mg/L for anaesthesia induction in mullet (Liza macrolepis) and Atlantic cod (Gadus morhua) (Barham et al. 1979; Mattson and Ripple 1989), while it reached 100–150 mg/L for tambaqui (Colossoma macropomum) as described by Gomes et al. (2001). Other findings claimed that MS-222 was potentially compromising the health of certain fish species such as crucian carp (Carassius auratus) reported by Cao et al. (2019), gilthead sea bream (Sparus aurata) as recorded by Teles et al. (2019) and also striped bass (Morone saxatilis) as mentioned by Kenter et al. (2019).
Povidone-iodine is a disinfectant frequently used in aquaculture, it is a type of iodophor, and it is composed of iodine as an oxidising agent and polyvinylpyrrolidone as a carrier to slowly release the free iodine into the water. Iodine is damaging the microorganisms by penetrating the cell wall, and then destroying the structural proteins and nucleic acid, as well as enzymes (Mcdonnell and Russell 1999; Mainous et al. 2010; Rico et al. 2014).
Overcrowding, agitation and scratches affecting fish during the transportation process, particularly for a long time, can induce severe stress with subsequent high mortality rate and increase the susceptibility to the bacterial disease by affecting the normal physiological functions, lowering fish immunity, impairing the function of natural parries represented in mucus (Ali et al. 2019) and also increasing bacterial multiplication and adherence to fish.
This work targeted to highlight the negative impact of long transportation on innate immune response particularly the effects on the skin mucus barrier functionality and also the stress response expressed in serum cortisol levels and clarify the beneficial role of tranquillizers and antiseptics in mitigating the stress response and disease susceptibility.
Materials and methods
Experimental design
Two hundred and forty apparently healthy male Nile tilapia (Oreochromis niloticus) were collected from a private fish farm (earthen pond) located at El Riad District, Kafr El-Sheikh Governorate. Fish were between 70 and 80 g in body weight, and were divided randomly into 4 equal groups (60 fish/group in 4 replicates, 15 fish/replicate). Fish transportation was performed according to the method of Noga (2010); each replicate (15 fish) was maintained in a labelled clear polyethylene bag filled to third with farm water at 25 °C and the other 2/3 with pure oxygen. Tricaine methanesulfonate (MS-222), Syncaine®, Syndel, Canada, was used as a tranquillizer at a dose of 40 mg/L (Noga, 2010) and Betadine® active ingredient povidone-iodine, 5%, Nile Company for Pharmaceuticals, Egypt, was used as an antiseptic at a dose of 20 ppm/L as indicated by Debuf (1991). Fish were grouped as follows:
-
G1: Fish were transported in farm water without using MS-222 or iodine.
-
G2: Fish were transported in farm water with 40 mg/L MS-222 as a tranquillizer.
-
G3: Fish were transported in farm water with 40 mg/L MS-222 as a tranquillizer plus 20 ppm/L iodine as antiseptic.
-
G4: Fish were transported in farm water with 20 ppm/L iodine as an antiseptic.
For simulating fish transportation during aquaculture practices or for performing any scientific experimental work using fish, the polyethylene bags containing fish were transported by a car for 2 h to the wet lab, Animal Health Research Institute, Kafr El-Sheikh. At 6 h after arrival to the laboratory, each replicate was placed in a separate glass aquarium with maintained aeration.
-
Replicate 1 (15 fish): Replicate 1 in each group was used for blood and mucus sample collection.
-
Replicates 2 and 3 (30 fish): Replicates 2 and 3 were used for calculating the cumulative mortality to avoid the non-specific deaths that may result from blood and mucus sampling.
-
Replicate 4 (15 fish): Replicate 4 was used in the challenge test.
Blood sampling
Blood samples were collected from 5 fishes in replicate 1 of each group at 0 h, 1 h, 6 h, 24 h, 48 h, 7 days and 14 days after arrival to the lab. Samples were according to the method described by Faulmann et al. (1983), as 0.5 mL of blood was collected from the caudal vessels of each fish. Serum samples were separated by centrifugation at 2000 rpm for 10 min.
Serum cortisol level
Serum cortisol level was assayed as a stress indicator marker; serum cortisol was extracted and estimated using the method described by Pickering and Pottinger (1983) and Wedemeyer (1970) using Ao Microplate Reader, Azure Biosystems, USA; absorbance was measured at 415 nm using 96-well cortisol ELISA kit, Cayman Chemical, USA. Serum glucose was spectrophotometrically determined according to the method mentioned by Trinder (1969) using Spinreact® glucose test kit.
Mucus analyses
Skin mucus collection
Five fish from each group were used for mucus collection, and each fish was placed in a polyethylene bag containing 10 mL of 50 mM NaCl, and then, the fish was gently rubbed inside the bag for 30 s. The collected solutions were centrifuged at 1500 rpm at 4 °C for 10 min, the supernatant was discarded, and the collected mucus was preserved at − 96 °C for further work.
Skin mucus lysozyme assays
Lysozyme activity of serum and skin mucus was calculated following the protocol of Parry et al. (1965). Briefly, 100 μL of skin mucus from each fish was loaded into 96-well plates, in triplicate, and then, 175 μL of Micrococcus luteus suspension was added. M. luteus suspension consists of 0.3 mg M. luteus (Sigma-Aldrich, USA), in 1 mL of 0.1 M citrate phosphate buffer, pH 5.8. The lysozyme activity expressed in μg/mL was measured by a microplate reader compared to standard. The turbidity changes were recorded at 540 nm every 30 s and for 10 min at 25 °C.
Skin mucus peroxidase activities
The peroxidase activity was measured following the methods described by Quade and Roth (1997). Briefly, 5 μL of skin mucus was loaded into 96 flat-bottomed plates in triplicate plus 45 μL of Hank’s Balanced Salt Solution (without Ca+2 or Mg+2) and 100 μL of solution 1. Solution 1 is prepared by addition of 10 μL of 30% H2O2 (Sigma-Aldrich, USA) to 40 mL of distilled water and then, one pill of 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich, USA) was added to the mixture. The peroxidase activity expressed in mU. Ml−1 was measured by the microplate reader at 405 nm.
Skin mucus antibacterial activities
Skin mucus antibacterial activity was determined according to Kumari et al. (2019). Equal volumes (100 μL) of O. niloticus mucus and sterile normal saline were vortex-mixed and placed (in triplicates) in the nutrient agar broth seeded with Aeromonas hydrophila bacterial suspension containing 106 CFU which is a virulent field strain previously isolated by Sherif and Abuleila (2022) and incubated for 1 h at 25 °C. A blank control was also prepared by replacing the mucus with sterile PBS. The mixture was then diluted with sterile PBS at a ratio of 1:10. The mucus-bacteria mixture (100 μL) was plated on blood agar, and the plates were incubated for 24 h at 27 °C. The number of viable bacteria was determined by counting the colonies grown on nutrient agar plates.
Fish food reflex
After 24 h of fish stocking in glass aquaria, floating fish feed was offered to each group and then repeated every 24 h; the food approach was assessed in the same manner indicated by Raby et al. (2012) and Samson et al. (2014).
Mortality rate
The number of dead fish in replicates 2 and 3 of each group was recorded daily for 14 days, and the cumulative mortality rate (CMR) was determined using the following equation:
Bacterial challenge
To determine the effect of using MS-222 and/or iodine for fish transportation on disease susceptibility to A. hydrophila, a bacterial challenge test was performed after 1 week of transportation using the cohabitation method (cohabitation of experimentally infected fish with healthy fish).
Before the challenge test, experimental infection for 10 acclimatised O. niloticus was performed using A. hydrophila (AHRAS2) virulent strain. Experimental infection was performed as described by Aboyadak et al. (2016) briefly, and each fish was intraperitoneally inoculated (I/P) with 0.2 mL of bacterial suspension containing 2.4 × 105 CFU/mL of A. hydrophila AHRAS2 strain. AHRAS2 strain was previously isolated by Sherif and Abuleila (2022), and deposited to GenBank under the accession number MW092007; it was positive for cytotoxic enterotoxin genes (act and alt). The LD50 value of A. hydrophila AHRAS2 for O. niloticus (50 ± 2.5 g) in body weight at 25 ± 1.5 °C was 0.2 mL of bacterial suspension containing 2.4 × 105 CFU/mL per fish.
After 1 week of fish transportation, G1 to G4 were challenged with A. hydrophila by cohabitation as described by Sherif et al. (2022); briefly, two clinically diseased fish (previously infected by I/P) were added to each aquaria containing replicate 4 for groups G1 to G4; then, the cumulative mortality rate was reported for another week. Each dead fish was considered only if A. hydrophila was re-isolated.
Statistical analyses
The obtained values were statistically analysed with SPSS software, SPSS Inc., Chicago, IL, USA (SPSS 2004), using analysis of two-way ANOVA. All values were expressed as the mean ± SE (standard error). Duncan’s multiple range test (Duncan 1955) was used to determine differences amongst groups and periods at a significance level of 0.05.
Biosafety procedure
This study followed the biosafety measures concerning the pathogen safety data sheets: infectious substances A. hydrophila, Pathogen Regulation Directorate, Public Health Agency of Canada (2011).
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed during this study.
Results
Serum cortisol level reached the maximum value in all groups at 6 h post transportation just before the ending of the transportation stress represented in high stocking density in the polyethylene bags (moving fish from bags to aquaria). The highest cortisol level was observed in group 1 which was transported without tranquillizer or antiseptic as it reached 6.36 μg/dL, followed by group 4 which was transported after the addition of iodine only (5.8 μg/dL), while the lowest value was observed for groups 2 and 3 that received MS-222 only and MS-222 + iodine in which cortisol level was 2.33 and 2.77 μg/dL respectively as represented in Table 1. Serum cortisol decreased nearly to the value previously reported at 0 h at 48 h post transportation before it returned to the normal value in all groups at 7 and 14 days post transportation as shown in Table 1.
Serum glucose concentration mostly followed the same pattern as plasma cortisol in which glucose concentration reached the maximum value in all groups at 6 h; fish in group 1 showed the highest glucose level (98 mg/dL) followed by group 4 by 89.83 mg/dL, while fish in groups 2 and 3 treated with MS-222 showed a nonsignificant increase in serum glucose in comparison with the non-treated group. Glucose level decreased significantly in all groups at 48 h post transportation except for group 4 and maintained near the normal value at 7 and 14 days post transportation as shown in Table 2.
Mucus lysozyme activity significantly decreased with time in all groups at 6 h when compared with the basal level at 0 h before the partial restoring of the activity at 48 h. Groups 2 and 3 treated with MS-222 tranquillizer showed high mucus lysozyme activity than the non-treated groups (1 and 4) as represented in Table 3. Mucus lysozyme activity was completely restored close to the normal values at the 7th day post transportation.
Mucus peroxidase activity was nonsignificantly decreased with the time after transportation before being restored to the baseline after 48 h post transportation; however, there was a nonsignificant change either within each group or between the different groups as represented in Table 4.
Mucus antibacterial activity was significantly higher in group 3 treated with MS-222 + iodine and group 4 treated with iodine alone than in the other 2 groups (G1 and G2); this effect was observed from 0 to 48 h but this effect was diminished at 7 and 14 days, and results showed a nonsignificant change between all of the experimental groups as represented in Table 5.
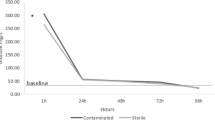
The cumulative mortality indicated that group 3 showed the lowest mortality rate (10%) followed by group 2 which showed 16.67% mortality and then group 4 with 20% mortality and finally group 1 with 33.33% mortality after an 8-h transportation (Figs. 1 and 2).
In Table 6, the experimental fish refuse to feed before 48 h post transportation while only groups 2 and 3 which were transported after tranquilization using MS-222 accepted to feed at 48 h but G1 and G4 started to feed at 72 h post transportation. All fish groups restored the normal feeding pattern (5% of body weight) on the 7th day post transportation.
The challenge test revealed that groups 2 and 3 that received the tranquillizer and/or iodine showed the lowest mortality rate (26.66%) followed by group 4 that received iodine only and finally group 1 which was transported in farm water without any medication as demonstrated in Fig. 3.
Discussion
Fish transportation is a stressful practice associated with the massive liberation of catecholamine (Wendelaar Bonga 1997; Toni et al. 2019); this process is resulting in high levels of plasma cortisol, glucose and other oxidative stress markers that have frequently served as stress indicators in fish (Morgan and Iwama 1997; Morgan et al. 1997). To maintain fish survival, welfare and performance, the practitioners tried to minimise the stress associated with fish transportation; this research is focusing on illustrating the deleterious effect of long transportation on Oreochromis niloticus and how to mitigate it by using MS-222 as a tranquillizer and/or iodine as an antiseptic during fish transportation either for aquaculture or for experimental work.
The present study results indicated that serum cortisol reached the maximum value at the end of the transportation process which indicated exposure of fish to severe stress, particularly groups not subjected to tranquillization; meanwhile, tranquillized O. niloticus using MS-222 with or without iodine showed significantly low cortisol level, and this finding can be attributed to decreased fish movement and low consciousness and so limited exposure to stress. In accordance, Pakhira et al. (2015) found that cortisol levels increased in Labeo rohita transported for 2.5 h, and McDonald and Milligan (1997) also reported increased blood cortisol concentration in response to stress conditions.
Serum glucose levels followed the same pattern as serum cortisol and reached the maximum value at 6 h; tranquillizer groups showed significantly lower serum glucose levels than the non-tranquillized groups; other researchers (Pakhira et al. 2015; Goes et al. 2017) reported similar findings for Labeo rohita and O. niloticus respectively. These could be owing to the effect of increased glucocorticoids represented in cortisol on glucose metabolism through induction of glycogenolysis and glyconeogenesis under the direct effect of cortisol (McDonald and Milligan 1997; Martínez-Porchas et al. 2009). Kuo et al. (2018) recorded that glucocorticoid promotes gluconeogenesis in the liver, but it decreased glucose uptake and utilization in skeletal muscle antagonising insulin response. Therefore, excess glucocorticoid induces hyperglycaemia.
Similarly, Cao et al. (2021) found that MS-222 and eugenol–anesthetised turbot (Scophthalmus maximus) has lower blood glucose and cortisol level than the control (non-anesthetised) groups. Navarro et al. (2016) also stated that plasma cortisol and glucose of the anaesthetized O. niloticus were lower than those of untreated controls. Different findings were observed by Oliveira et al. (2009); they found that O. niloticus anaesthetized using MS-222 had higher plasma glucose compared to the control. These different results could be due to differences in experimental conditions or due to exposure of fish to stress before anaesthesia. Félix et al. (2021) described nonsignificant increased cortisol levels in O. niloticus anaesthetized with MS-222 at a dose of 40 mg/L and then transported for 6 h, indicating the efficacy of fish anaesthesia and tranquilization in lowering the plasma cortisol. The present research results proved that tranquillized O. niloticus either with or without iodine treatment started to restore their normal physiological condition after 48 h of transportation that was following Félix et al. (2021) as they recorded the normalisation of physiological responses of anaesthetized O. niloticus, compared to the control; similarly, Teles et al. (2019) claimed that anaesthetics can lessen the impacts of transport stress.
A wide range of non-specific immunity parameters is found in fish mucus, including lysozymes, peroxidases and immunoglobulins (Guardiola et al. 2014). The present research results indicated that tranquillized O. niloticus showed the highest level of lysozyme and peroxidase activities compared with the other 2 groups. Profuse mucus secretion is mainly produced in response to stress conditions during fish transportation, while non-stressed tranquillized fish produced normal thick mucus reach in lysozymes and peroxidases. In harmony with these results, Cao et al. (2019) and Kenter et al. (2019) conclude that MS-222 might stimulate the immune biochemical responses in different fish species subjected to transport. Both iodine-treated groups showed the maximum mucus antibacterial activity in comparison with iodine non-treated groups. In our point of view, this result can be attributed to the presence of iodine which is absorbed to fish bodies from the water and excreted in mucus. Iodine has antibacterial activity against many bacterial pathogens. Mucus also contains immunoglobulins and lysozymes which have also antibacterial action, so groups 1 and 2 showed a considerable in vitro antibacterial activity against A. hydrophila but the presence of iodine is potentiating this action. Accordingly, Chen et al. (2018) indicated that 10 ppm and 20 ppm of povidone-iodine effectively killed 99 and 100% of A. hydrophila in vitro and protected swamp eel (Monopterus albus).
Both of the tranquilized O. niloticus groups (with or without iodine) started to consume feed after 2 days and then partially restored normal food reflex after 3 days post transportation which was earlier than the other groups due to the protective effect of MS-222 against the transportation stress which induced a rapid recovery. All groups feed normally (by consuming about 5% of their live body weight) on the 7th day which indicated the complete restoration of the normal physiological functions at this time. This observation was similar to Wendelaar Bonga’s (1997) findings; he stated that under stress conditions, the metabolic energy of fish is reallocated away from growth and reproduction towards restoring homeostasis (respiration, locomotion, hydromineral regulation and tissue repair) and these activities are extended to the recovery phase after stress. Similarly, Fernandes and Volpato (1993) stated that the food consumption of fish was decreased under chronic stress, and Pickering and Stewart (1984) also reported that food consumption of brown trout Salmo trutta (L.) was significantly decreased under the stress induced by crowdedness.
A high mortality rate occurred in fish within the first 24 h after transportation, which could be due to homeostasis disturbances and skin abrasion (Gomes et al. 2003), and stressed fish suffered from skin abrasions that could open the gate to the bacterial infection (Stickney 2005). The cumulative mortality rate after the transportation process clarified that the iodine-tranquillized group showed the lowest mortality rate amongst all groups; this could be attributed to the augmented effective role of MS-222 plus the antibacterial effect of iodine which protected fish from accidental bacterial infection during transportation and also decreased the pathogenic bacterial load and improved fish health status. Using iodine at a low dose as 100 ppm for a minimum 10 min of exposure time can effectively destruct germs (OIE 2009; Heiner et al. 2010). Chatchawanchonteera et al. (2015) reported that povidone-iodine at a dose of 100 ppm for 15-min exposure at 37 °C could inactivate 6 logs of S. agalactiae isolated from fish. Group 2 (transported in MS-222 only) showed a lower mortality rate in comparison with the non-tranquillized groups (1 and 4); this highlighted the beneficial role of MS-222 as a tranquillizer which protected fish from the harmful effect of stress associated with transportation by maintaining fish calm (not exposed to friction or scratches) and also decreasing fish excitation and subsequently fish metabolic rate that preserved fish energy and finally kept the immune system functionally active (kept mucus lysozymes and peroxidases higher than in the non-tranquillized groups). Group 4 (iodine-treated group without tranquillizer) showed a low mortality rate than the control group (group 1), further proving the effective role of iodine in protecting fish during the transportation process from bacterial infection.
Infectious diseases are amongst the major obstacles to expansion in the aquaculture industry (Mishra et al. 2018; Sherif et al. 2020, 2021). In this study, cohabitation challenge with A. hydrophila showed that both of the tranquillized O. niloticus showed low mortality rates than the other 2 groups; this indicated higher resistance to bacterial infection due to rapid restoration of the normal physiological functions of the immune system and also due to the protective role of mucus lysozymes and peroxidases. Accordingly, Esteban (2012), Guardiola et al. (2014), Hoseinifar et al. (2019), and Somamoto and Nakanishi (2020) reported that skin mucus has a vital molecule of the non-specific immune system and acts as the first protective layer which stands against pathogen infection; it contains a wide range of biological substances, such as proteins, lysozyme, immunoglobulin and lectins (Minniti et al. 2017; Reverter et al. 2018). The lysozyme activity can act as a non-specific molecule that beneficially protects the fish from infectious disease through the breakdown of 1,4 glycosidic bonds present in the peptidoglycan of both Gram-positive and Gram-negative bacterial cell walls (Subramanian et al. 2007; Saurabh and Sahoo 2008).
Conclusion
Using MS-222 as a tranquillizer plus povidone-iodine as an antiseptic during fish transportation during aquaculture practices has a beneficial role in decreasing stress response and subsequently improving fish performance and helping in rapid restoration of the normal physiology functions (rabid recovery), maintaining a protective level of immune status and helping in bacterial disease resistance.
Data availability
Data are available on request from the authors.
Code availability
Not applicable.
Change history
04 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10499-022-01011-5
References
Aboyadak IM, Ali NG, Abdel-Aziz MM, Gado MS, El-Shazly KA (2016) Role of some antibacterial drugs in control Streptococcus iniae infection in Oreochromis niloticus. Int J Pharm Clin Res 1(5):555573. https://doi.org/10.19080/JPCR.2016.01.555573
Ali NGM, Aboyadak IM, El-Sayed HS (2019) Chemotherapeutic control of Gram-positive infection in white sea bream (Diplodus sargus, Linnaeus 1758) broodstock. Vet World 12(2):316–324. https://doi.org/10.14202/vetworld.2019.316-324
Barham WT, Caiger KM, Visser JGJ (1979) The use of benzocaine hydrochloride as fish tranquillizer and anaesthetic in saline waters. Afr J Aquat Sci 5(2):94–96
Brinchmann MF (2016) Immune relevant molecules identified in the skin mucus of fish using-omics technologies. Mol Biosyst 12(7):2056–2063
Cao J, Wang Q, Qiu W, Mei J, Xie J (2021) Transport and recovery of turbot (Scophthalmus maximus) sedated with MS-222 and eugenol: effects on intermediary metabolism and osmoregulation. Animals 11(8):2228
Cao X, Wang Y, Yu N, Le Q, Hu J, Yang Y, ... Yan X (2019) Transcriptome analysis reveals the influence of anaesthetic stress on the immune system of crucian carp (Carassius auratus) under the process of treatment and low concentration transport by MS‐222 and Eugenol. Aquac Res 50(11): 3138–3153
Carda-Diéguez M, Ghai R, Rodríguez-Valera F, Amaro C (2017) Wild eel microbiome reveals that skin mucus of fish could be a natural niche for aquatic mucosal pathogen evolution. Microbiome 5(1):1–15. https://doi.org/10.1186/s40168-017-0376-1
Chatchawanchonteera A, Kitcharoenpunya R, Nasri T, Chaichana T, Ronkhakulpipat N, Rattanapratumchai C (2015) In vitro study on efficacy of disinfectants against Streptococcus agalactiae. The 6th Hat Yai National Conference. Hat Yai University, Hat Yai, Thailand, pp 1535–1543
Chen X, Lai C, Wang Y, Wei L, Zhong Q (2018) Disinfection effect of povidone-iodine in aquaculture water of swamp eel (Monopterus albus). Peer J 6:e5523. https://doi.org/10.7717/peerj.5523
Chen SW, Liu CH, Hu SY (2019) Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 84:695–703. https://doi.org/10.1016/j.fsi.2018.10.059
Debuf Y (1991) The veterinary formulary handbook of medicines used in veterinary practice. J S Afr Vet Assoc 62(4):181
Duncan DB (1955) Multiple range and multiple -F test. Biometrics 11:10
Esteban MÁ (2012) A review: An overview of the immunological defenses in fish skin. ISRN Immunol 1–29. https://doi.org/10.5402/2012/853470
FAO (2018) Food and Agriculture Organization, The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals. Italy, FAO, Rome
Faulmann E, Cuchens MA, Lobb CJ, Miller NW, Clem LW (1983) An effective culture system for studying in vitro mitogenic responses of channel catfish lymphocytes. Trans Am Fish Soc 112(5):673–679. https://doi.org/10.1577/1548-8659(1983)112%3c673:AECSFS%3e2.0.CO;2
Félix L, Correia R, Sequeira R, Ribeiro C, Monteiro S, Antunes L, ... Valentim A (2021) MS-222 and propofol sedation during and after the simulated transport of Nile tilapia (Oreochromis niloticus). Biology 10(12):1309
Fernandes MO, Volpato GL (1993) Estresse social ecrescimento em peixes. Anais De Etologia 11:129–141
Goes ESR, de Lara AF, Gasparino E, Goes MD, Zuanazzi JSG, Lopera-Barrero NM, Rodriguez MPR, Castro PL, Ribeiro RP (2017) Effects of transportation stress on quality and sensory profiles of Nile tilapia fillets. Scientia Agricola 75(4):321–328. https://doi.org/10.1590/1678-992X-2016-0387
Gomes LC, Chippari-Gomes AR, Lopes NP, Roubach R, Araújo Lima CAR (2001) Efficacy of benzocaine as an anesthetic in juvenile tambaqui Colossoma macropomum. J World Aquac Soc 32(1):426–431
Gomes LC, Roubach R, Araujo-Lima CA, Chippari-Gomes AR, Lopes NP, Urbinati EC (2003) Effect of fish density during transportation on stress and mortality of juvenile tambaqui Colossoma macropomum. J World Aquac Soc 34(1):76–84. https://doi.org/10.1111/j.1749-7345.2003.tb00041.x
Guardiola FA, Cuesta A, Arizcun M, Meseguer J, Esteban MA (2014) Comparative skin mucus and serum humoral defence mechanisms in the teleost gilthead seabream (Sparus aurata). Fish Shellfish Immunol 36(2):545–551
Heiner JD, Hile DC, Demons ST, Wedmore IS (2010) 10% Povidone-iodine may be a practical field water disinfectant. Wilderness Environ Med 21:332–336. https://doi.org/10.1016/j.wem.2010.09.008
Hoseinifar SH, Sohrabi A, Paknejad H, Jafari V, Paolucci M, Van Doan H (2019) Enrichment of common carp (Cyprinus carpio) fingerlings diet with Psidium guajava: the effects on cutaneous mucosal and serum immune parameters and immune related genes expression. Fish Shellfish Immunol 86:688–694. https://doi.org/10.1016/j.fsi.2018.12.001
Kenter LW, Gunn MA, Berlinsky DL (2019) Transport stress mitigation and the effects of preanesthesia on striped bass. N Am J Aquac 81(1):67–73. https://doi.org/10.1002/naaq.10072
Kuo T, McQueen A, Chen TC, Wang JC (2018) Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol 872:99–126. https://doi.org/10.1007/978-1-4939-2895-8_5
Kumari S, Tyor AK, Bhatnagar A (2019) Evaluation of the antibacterial activity of skin mucus of three carp species. Int Aquat Res. 11:225–239. https://doi.org/10.1007/s40071-019-0231-z
Mainous ME, Smith SA, Kuhn DD (2010) Effect of common aquaculture chemicals against Edwardsiella ictaluri and E. tarda. J Aquat Anim Health 22:224–228. https://doi.org/10.1577/H10-020.1
Martínez-Porchas M, Martínez-Córdova LR, Ramos-Enriquez R (2009) Cortisol and glucose: reliable indicators of fish stress. Pan-Am J Aquat Sci 4(2):158–178
Mattson NS, Ripple TH (1989) Metomidate, a better anesthetic for cod (Gadus morhua) in comparison with benzocaine, MS-222, chlorobutanol, and phenoxyethanol. Aquaculture 83(1–2):89–94
McDonald G, Milligan CL (1997) Ionic, osmotic and acid-base regulation in stress. In: Iwama GW, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture. University Press, Cambridge, UK, pp 119–144
Mcdonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179
Minniti G, Hagen LH, Porcellato D, Jørgensen SM, Pope PB, Vaaje-Kolstad G (2017) The skin-mucus microbial community of farmed Atlantic salmon (Salmo salar). Front Microbiol 8:2043. https://doi.org/10.3389/fmicb.2017.02043
Mishra A, Nam GH, Gim JA, Lee HE, Jo A, Kim HS (2018) Current challenges of Streptococcus infection and effective molecular, cellular, and environmental control methods in aquaculture. Mol Cells 41(6):495
Morgan JD, Iwama GK (1997) Measurements of stressed states in the field. In: Iwana GW, Pickering AD, Sumpter JP (eds) Fish stress and health in aquaculture. University Press, Cambridge, UK, pp 247–270
Morgan JD, Sakamoto T, Grau EG, Iwama GK (1997) Physiological and respiratory responses of the Mozambique tilapia (Oreochromis mossambicus) to salinity acclimation. Comp Biochem Physiol 117(3):391–398
Navarro RD, França RP, Paludo GR, Bizarro YWS, da Silva RF, Navarro FKSP (2016) Physiological and hematological responses of Nile tilapia (Oreochromis Niloticus) to different anesthetics during simulated transport conditions. Acta Sci Technol 38(3):301–306. https://doi.org/10.4025/actascitechnol.v38i3.28377
Noga EJ (2010) Pharmacopoeia. In: Fish disease: diagnosis and treatment. 2nd edn. John Wiley & Sons, pp 375–420
OIE, World Organization for Animal Health (2009) Methods for disinfection of aquaculture establishments. In Manual of diagnostic tests for aquatic animals (pp. 31–42). Retrieved from http://www.oie.int/fileadmin/Home/eng/Health_standards/aahm/2009/1.1.3_DISINFECTION.pdf
Oliveira JR, Carmo JL, Oliveira KKC (2009) Cloreto de sódio, benzocaína e óleo de cravo-da-índia na água de transporte de tilápia-do-Nilo. Rev Bras De Zootec 38(7):1163–1169. https://doi.org/10.1590/S1516-35982009000700001
Pakhira C, Nagesh TS, Abraham TJ, Dash G, Behera S (2015) Stress responses in rohu, Labeo rohita transported at different densities. Aquac Rep 2:39–45. https://doi.org/10.1016/j.aqrep.2015.06.002
Park MO, Hur WJ, Im SY, Seol DW, Lee J, Park IS (2008) Anaesthetic efficacy and physiological responses to clove oil anaesthetized kelp grouper Epinephelus bruneus. Aquac Res 39(8):877–884. https://doi.org/10.1111/j.1365-2109.2008.01941.x
Park IS, Gil HW, Lee TH, Nam YK, Lim SG, Kim DS (2017) Effects of clove oil and lidocaine-HCl anesthesia on water parameter during simulated transportation in the marine medaka, Oryzias dancena. Dev Reprod 21(1):19–33. https://doi.org/10.12717/DR.2017.21.1.019
Parry RM Jr, Chandan RC, Shahani KM (1965) A rapid and sensitive assay of muramidase. SEBM 119(2):384–386. https://doi.org/10.3181/00379727-119-30188
Pickering AD, Pottinger P (1983) Seasonal and diet changes in plasma cortisol levels of the brown trout, Salmo trutta L. Gen Corn Endocrinol 49:232–239
Pickering AD, Stewart A (1984) Acclimation of interrenal tissue of the brown trout, Salmo trutta L. to chronic crowding stress. J Fish Biol 24:731–740. https://doi.org/10.1111/j.1095-8649.1984.tb04844.x
Popovic TN, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, PersinBerakovic A, SauerbornKlobucar R (2012) Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J Appl Ichthyol 28(4):553–564. https://doi.org/10.1111/j.1439-0426.2012.01950.x
Prabu E, Rajagopalsamy CBT, Ahilan B, Jeevagan IJMA, Renuhadevi M (2019) Tilapia–an excellent candidate species for world aquaculture: a review. Annu Res Rev Biol 1–14. https://doi.org/10.9734/arrb/2019/v31i330052
Public Health Agency of Canada (2011) Pathogen safety data sheets: infectious substances – Aeromonas hydrophila. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/aeromonas-hydrophila.html
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58(3–4):239–248. https://doi.org/10.1016/S0165-2427(97)00048-2
Raby GD, Donaldson MR, Hinch SG, Patterson DA, Lotto AG, Robichaud D, ... Cooke SJ (2012) Validation of reflex indicators for measuring vitality and predicting the delayed mortality of wild coho salmon bycatch released from fishing gears. J Appl Ecol 49(1):90–98. https://doi.org/10.1111/j.1365-2664.2011.02073.x
Reverter M, Tapissier-Bontemps N, Lecchini D, Banaigs B, Sasal P (2018) Biological and ecological roles of external fish mucus: a review. Fishes 3(4):41
Rico A, Oliveira R, McDonough S, Matser A, Khatikarn J, Satapornvanit K, ... Van den Brink PJ (2014) Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ Pollut 191:8–16. https://doi.org/10.1016/j.envpol.2014.04.002
Sampaio FD, Freire CA (2016) An overview of stress physiology of fish transport: changes in water quality as a function of transport duration. Fish Fish 17(4):1055–1072. https://doi.org/10.1111/faf.12158
Samson E, Brownscombe JW, Cooke SJ (2014) Behavioural and reflex responses of mottled mojarra Eucinostomus lefroyi (Gerreidae) to cold shock exposure. Aquat Biol 23(1):101–108. https://doi.org/10.3354/ab00609
Sanahuja I, Fernández-Alacid L, Ordóñez-Grande B, Sánchez-Nuño S, Ramos A, Araujo RM, Ibarz A (2019) Comparison of several non-specific skin mucus immune defences in three piscine species of aquaculture interest. Fish Shellfish Immunol 89:428–436. https://doi.org/10.1016/j.fsi.2019.04.008
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39(3):223–239. https://doi.org/10.1111/j.1365-2109.2007.01883.x
Sherif AH, AbuLeila RH (2022) Prevalence of some pathogenic bacteria in caged- Nile tilapia (Oreochromis Niloticus) and their possible treatment. Jordan J Biol Sci. 15(2):239–247. https://doi.org/10.54319/jjbs/150211
Sherif AH, Gouda MY, Naena NA, Ali AH (2020) Alternate weekly exchanges of feeding regime affect the diversity of intestinal microbiota and immune status of Nile tilapia Oreochromis niloticus. Aquac Res 51(10):4327–4339. https://doi.org/10.1111/are.14778
Sherif AH, Gouda M, Darwish S, Abdelmohsin A (2021) Prevalence of antibiotic-resistant bacteria in freshwater fish farms. Aquac Res 52(5):2036–2047. https://doi.org/10.1111/are.15052
Sherif AH, Abdelsalam M, Ali NG, Mahrous KF (2022) Zinc oxide nanoparticles boost the immune responses in Oreochromis niloticus and improve disease resistance to Aeromonas hydrophila infection. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03183-w
Simões LN, Lombardi DC, Gomide A, Gomes LC (2011) Efficacy of clove oil as anesthetic in handling and transportation of Nile tilapia, Oreochromis niloticus (Actinopterygii: Cichlidae) juveniles. Zoologia (curitiba) 28(3):285–290
Somamoto T, Nakanishi T (2020) Mucosal delivery of fish vaccines: local and systemic immunity following mucosal immunisations. Fish Shellfish Immunol 99:199–207. https://doi.org/10.1016/j.fsi.2020.01.005
SPSS (2004) Statistical and Package for Social Science, SPSS for Windows Release 14.0.0, 19 June, 2004. Standard Version. copyright SPSS Inc., p. 1989–2004
Stickney RR (2005) The Healthy Fish is a Happy Fish. In: Aquaculture: an introductory text, 1st edn. CABI Publishing UK, pp 132–153.
Subramanian S, MacKinnon SL, Ross NW (2007) A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp Biochem Physiol B Biochem Mol Biol 148(3):256–263. https://doi.org/10.1016/j.cbpb.2007.06.003
Sumpter JP, Pickering AD, Pottinger TG (1985) Stress-induced elevation of plasma α-MSH and endorphin in brown trout. Salmo Trutta l Gen Comp Endocrinol 59(2):257–265. https://doi.org/10.1016/0016-6480(85)90377-6
Teles M, Oliveira M, Jerez-Cepa I, Franco-Martínez L, Tvarijonaviciute A, Tort L, Mancera JM (2019) Transport and recovery of gilthead sea bream (Sparus aurata L.) sedated with clove oil and ms222: effects on oxidative stress status. Front Physiol. 10:523. https://doi.org/10.3389/fphys.2019.00523
Toni M, Manciocco A, Angiulli E, Alleva E, Cioni C, Malavasi S (2019) Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animals 13:161–170. https://doi.org/10.1017/S1751731118000940
Trinder P (1969) Serum glucose determination. Ann. Clin. Biochem., 6:24. Cited from Boehringer Mannheim.Gmth Diagnostickit. https://doi.org/10.1177/000456326900600108
Wedemeyer GA (1970) The role of stress in the disease resistance of fishes. Spec Publ Am Fish Soc 5:30–35. https://doi.org/10.1152/physrev.1997.77.3.591
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this work. All authors analysed and interpreted the data regarding gene expression and enzymes. All authors performed the experimental study and were major contributors to writing the manuscript. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sherif, A.H., Eldessouki, E.A., Sabry, N.M. et al. The protective role of iodine and MS-222 against stress response and bacterial infections during Nile tilapia (Oreochromis niloticus) transportation. Aquacult Int 31, 401–416 (2023). https://doi.org/10.1007/s10499-022-00984-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00984-7