Abstract
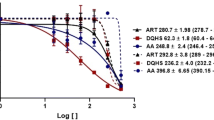
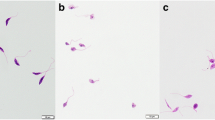
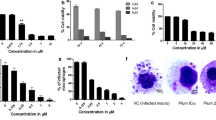
Natamycin, a Food and Drug Administration approved anti-fungal drug, and also used as a food additive was evaluated for anti-leishmanial activity since it is known to specifically bind to ergosterol, which is essential to these parasites but absent in mammals. Promising anti-proliferative activity was observed in both promastigote and amastigote forms of the parasite with IC50 values of 15 and 8 µM respectively and a selective index of 12.5. The ultrastructural effects of natamycin on both forms of the parasite and physiological effects on promastigotes were studied in detail for the first time. Electron microscopic observations in treated cells revealed sub-cellular changes like plasma membrane alterations, accumulation of vesicles in the flagellar pocket and extensive mitochondrial damage. Natamycin treatment in promastigotes resulted in elevation of cytosolic calcium (Ca2+) levels which caused irreversible loss of mitochondrial membrane potential. This resulted in depletion of cellular ATP levels along with ROS generation finally leading to apoptosis-like and necrotic cell death. In view of our observations along with the safety profile of an existing anti-fungal drug, natamycin may be further investigated for repurposing it as a promising drug candidate against Leishmaniasis.









Similar content being viewed by others
References
Swaminath CS, Shortt HE, Anderson LAP (2006) Transmission of Indian kala-azar to man by the bites of Phlebotomus argentipes, ann and brun. Indian J Med Res 123:473–477
Torres-guerrero E, Quintanilla-cedillo MR, Ruiz-esmenjaud J, Arenas R (2017) Leishmaniasis: a review. F1000Res 6:1–15. https://doi.org/10.12688/f1000research.11120.1
NVBDCP|National Vector Borne Disease Control Programme. http://nvbdcp.gov.in/ka-cd.html. Accessed 29 Dec 2015
Singh N, Kumar M, Singh RK (2012) Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Med 5:485–497. https://doi.org/10.1016/S1995-7645(12)60084-4
Atta HM, Selim SsM, Zayed MS (2012) Natamycin antibiotic produced by Streptomyces sp.: fermentation, purification and biological activities. J Am Sc 8:2
Pimaricin N (2015) Safety evaluation of certain food additives and contaminants. WHO, Geneva
Austin A, Lietman T, Rose-nussbaumer J (2017) Update on the management of infectious keratitis. Ophthalmology 124(11):1678–1689
Barrett MP, Croft SL (2012) Management of trypanosomiasis and leishmaniasis. Br Med Bull 104:175–196. https://doi.org/10.1093/bmb/lds031
Beach DH, Goad LJ, Holz GG (1988) Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol Biochem Parasitol 31:149–162
Johnston JB, Chen S, Kellar D, Siqueira-neto JL (2015) Targeting ergosterol biosynthesis in Leishmania donovani: essentiality of sterol 14alpha-demethylase. PLoS Negl Trop Dis 9(3):1–17. https://doi.org/10.1371/journal.pntd.0003588
Maria Y, Jones L, Leeuwen MR, Van et al (2010) Natamycin inhibits vacuole fusion at the priming phase via a specific interaction with ergosterol. Antimicrob Agents Chemother 54:2618–2625. https://doi.org/10.1128/AAC.01794-09
Tanner W (2014) Membrane transport inhibition as mode of action of polyene antimycotics: recent data supported by old ones. Food Technol Biotechnol 52:8–12
Van Leeuwen MR, Golovina EA, Dijksterhuis J (2009) The polyene antimycotics nystatin and filipin disrupt the plasma membrane, whereas natamycin inhibits endocytosis in germinating conidia of Penicillium discolor. J Appl Microbiol 106:1908–1918. https://doi.org/10.1111/j.1365-2672.2009.04165.x
Raina P, Kaur S (2006) Chronic heat-shock treatment driven differentiation induces apoptosis in Leishmania donovani. Mol Cell Biochem 289:83–90. https://doi.org/10.1007/s11010-006-9151-5
Das M, Mukherjee SB, Shaha C (2001) Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J Cell Sci 114:2461–2469
Paris C, Loiseau PM, Bories C (2004) Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother 48:852–859. https://doi.org/10.1128/AAC.48.3.852
Sen N, Das BB, Ganguly A et al (2004) Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ 11:924–936. https://doi.org/10.1038/sj.cdd.4401435
Houghton P, Fang R, Techatanawat I et al (2007) The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 42:377–387. https://doi.org/10.1016/j.ymeth.2007.01.003
Awasthi BP, Kathuria M, Pant G, Kumari N (2016) Plumbagin, a plant-derived naphthoquinone metabolite induces mitochondria mediated apoptosis-like cell death. Apoptosis 21(8):941–953. https://doi.org/10.1007/s10495-016-1259-9
Kathuria M, Bhattacharjee A, Sashidhara KV et al (2014) Induction of mitochondrial dysfunction and oxidative stress in Leishmania donovani by orally active clerodane diterpene. Antimicrob Agents Chemother 58:5916–5928. https://doi.org/10.1128/AAC.02459-14
Carvalho L, Martínez-garcía M, Pérez-victoria I et al (2015) The oral antimalarial drug tafenoquine shows activity against Trypanosoma brucei. Antimicrob Agents Chemother 59:6151–6160. https://doi.org/10.1128/AAC.00879-15
Philosoph H, Zilberstein D (1989) Regulation of intracellular calcium in promastigotes of the human protozoan parasite Leishmania donovani. J Biol Chem 264(18):10420–10424
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833
Smirlis D, Duszenko M, Ruiz AJ, Scoulica E, Bastien P, Fasel N, Soteriadou K (2010) Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasit Vectors 3:107. https://doi.org/10.1186/1756-3305-3-107
Vannier-santos MA, Urbina JA, Martiny A (1995) Alterations induced by antifungal compounds ketoconazole and terbinafine in Leishmania. J Eukaryot Microbiol 42:337–346
Rodrigues JCF, Rodriguez C, Urbina JA (2002) Ultrastructural and biochemical alterations induced by 22,26-azasterol, a delta(24(25))-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob Agents Chemother 46:487–499. https://doi.org/10.1128/AAC.46.2.487
Welscher YM, Napel HH, Masia M et al (2008) Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J Biol Chem 283:6393–6401. https://doi.org/10.1074/jbc.M707821200
Goad LJ, Holz GG, Beach DH (1984) Sterols of Leishmania species. implications for biosynthesis. Mol Biochem Parasitol 10:161–170
Channel C (2018) Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane. Antimicrob Agents Chemother 62:1. https://doi.org/10.1128/AAC.01614-17
García-marchan Y, Fernandez A, Rodriguez N et al (2009) Amiodarone destabilizes intracellular Ca 2 ϩ homeostasis and biosynthesis of sterols in Leishmania mexicana. Antimicrob Agents Chemother 53:1403–1410. https://doi.org/10.1128/AAC.01215-08
Benaim G, Casanova P, Hernandez-rodriguez V et al (2014) Dronedarone, an amiodarone analog with improved anti-Leishmania mexicana efficacy. Antimicrob Agents Chemother 58:2295–2303. https://doi.org/10.1128/AAC.01240-13
Corral MJ, Benito-pe E, Jiménez-antón MD (2016) Allicin induces calcium and mitochondrial dysregulation causing necrotic death in Leishmania. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0004525
Mukherjee SB, Das M, Sudhandiran G, Shaha C (2002) Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J Biol Chem 277:24717–24727. https://doi.org/10.1074/jbc.M201961200
Majumder HK, Nac ROSÁ, Calcium Á (2008) Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13:867–882. https://doi.org/10.1007/s10495-008-0224-7
Carvalho L, Roma J, Lo C et al (2011) The 8-aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovani promastigotes by targeting succinate dehydrogenase. Antimicrob Agents Chemother 55:4204–4210. https://doi.org/10.1128/AAC.00520-11
Schneider A (2001) Unique aspects of mitochondrial biogenesis in trypanosomatids. Int J Parasitol 31(13):1403–1415
Tomás AM, Castro H (2013) Redox metabolism in mitochondria of trypanosomatids. Antioxid Redox Signal 19(7):696–707. https://doi.org/10.1089/ars.2012.4948
Luque-Ortega JR, Rivas L (2007) Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob Agents Chemother 51(4):1327–1332
Saugar JM, Delgado J, Hornillos V, Luque-Ortega JR, Amat-Guerri F, Acuña AU, Rivas L (2007) Synthesis and biological evaluation of fluorescent leishmanicidal analogues of hexadecylphosphocholine (miltefosine) as probes of antiparasite mechanisms. J Med Chem 50(24):5994–6003
Dey R, Meneses C, Salotra P, Kamhawi S, Nakhasi HL, Duncan R (2010) Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol Microbiol 77(2):399–414
Roy A, Ganguly A, BoseDasgupta S et al (2008) Mitochondriadependent reactive oxygen species-mediated programmed cell death induced by 3,30-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol 74:1292–1307. https://doi.org/10.1124/mol.108.050161
Villa-Pulgarín JA, Gajate C, Botet J, Jimenez A, Justies N, Varela-M RE, Cuesta-Marbán Á, Müller I, Modolell M, Revuelta JL, Mollinedo F (2017) Mitochondria and lipid raft-located FOF1-ATP synthase as major therapeutic targets in the antileishmanial and anticancer activities of ether lipid edelfosine. PLoS Negl Trop Dis 11(8):e0005805. https://doi.org/10.1371/journal.pntd.0005805
BoseDasgupta S, Das SS, Sengupta S, Ganguly A, Roy A, Dey S et al (2008) The caspase independent algorithm of programmed cell death in Leishmania induced by baicalein: the role of LdEndoG, LdFEN-1 and LdTatD as a DNA ‘degradosome’. Cell Death Differ 15:1629–1640
Mittal N, Muthuswami R, Madhubala R (2017) The mitochondrial SIR2 related protein 2 (SIR2RP2) impacts Leishmania donovani growth and infectivity. PLoS Negl Trop Dis 11(5):e0005590. https://doi.org/10.1371/journal.pntd.0005590
Dolai S, Yadav RK, Pal S, Adak S (2009) Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot Cell 8(11):1721–1731. https://doi.org/10.1128/EC.00198-09
Kumar A, Das S, Purkait B, Sardar AH, Ghosh AK, Dikhit MR, Abhishek K, Das P (2014) Ascorbate peroxidase, a key molecule regulating amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 58(10):6172–6184. https://doi.org/10.1128/AAC.02834-14
Paramchuk WJ, Ismail SO, Bhatia A, Gedamu L (1997) Cloning, characterization and overexpression of two iron superoxide dismutase cDNAs from Leishmania chagasi: role in pathogenesis. Mol Biochem Parasitol 90(1):203–221
Veiga-santos P, Li K, Lameira L et al (2015) SQ109, a new drug lead for chagas disease. Antimicrob Agents Chemother 59:1950–1961. https://doi.org/10.1128/AAC.03972-14
Oldfield E, Benaim G (2016) Inhibition of Leishmania mexicana growth by the tuberculosis drug SQ109. Antimicrob Agents Chemother 60:6386–6389. https://doi.org/10.1128/AAC.00945-16
Cola J, Rodrigues F, Concepcion JL et al (2008) In vitro activities of ER-119884 and E5700, two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrob Agents Chemother 52:4098–4114. https://doi.org/10.1128/AAC.01616-07
Webster P, Russell G (1993) The flagellar pocket of Trypanosomatids. Parasitol Today 9:201–206
Menna-barreto RFS, Goncalves RLS, Costa EM et al (2009) The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic Biol Med 47:644–653. https://doi.org/10.1016/j.freeradbiomed.2009.06.004
Sidana A, Negi AK, Farooq U (2017) In vitro assessment of natamycin and nystatin antileishmanial activity of natamycin and nystatin. Braz Arch Biol Technol 60:1–7
Arima AA, Pavinatto FJ, Oliveira ON, Gonzales ERP (2014) The negligible effects of the antifungal natamycin on cholesterol-dipalmitoyl phosphatidylcholine monolayers may explain its low oral and topical toxicity for mammals. Colloids Surf B 122:202–208
Acknowledgements
Funding from CSIR network project HOPE (BSC0114) is acknowledged. BA is recipient of ICMR Senior Research fellowship. We sincerely thank Dr. A.A. Sahasrabuddhe, Dr. Neena Goyal and Dr. Susanta Kar for generously sharing MHOM/IN/80/DD8, luciferase transfected Leishmania promastigotes and J774.1 macrophages. We thank Mr. A.L. Vishwakarma for assistance in flow cytometry experiments and acknowledge CSIR–CDRI SAIF flow cytometry facility. Garima Pant and Madhuli Srivastava are acknowledged for technical assistance during EM experiments. CDRI communication number is 9705.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Awasthi, B.P., Mitra, K. In vitro leishmanicidal effects of the anti-fungal drug natamycin are mediated through disruption of calcium homeostasis and mitochondrial dysfunction. Apoptosis 23, 420–435 (2018). https://doi.org/10.1007/s10495-018-1468-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1468-5