Abstract
The Eurasian shrew and vole tick Ixodes trianguliceps Birula lives in the nests and burrows of its small mammalian hosts and is—along with larvae and nymphs of Ixodes ricinus or Ixodes persulcatus—one of the most commonly collected tick species from these hosts in its Eurasian range. Ixodes trianguliceps is a proven vector of Babesia microti. In this study, up-to-date maps depicting the geographical distribution and the climate preference of I. trianguliceps are presented. A dataset was compiled, resulting in 1161 georeferenced locations in Eurasia. This data set covers the entire range of I. trianguliceps for the first time. The distribution area between 8\(^\circ\) W–105\(^\circ\) E and 40–69\(^\circ\) N extends from Northern Spain to Western Siberia. To investigate the climate adaptation of I. trianguliceps, the georeferenced locations were superimposed on a high-resolution map of the Köppen–Geiger climate classification. The Köppen profile for I. trianguliceps, i.e., a frequency distribution of the tick occurrence under different climates, shows two peaks related to the following climates: warm temperate with precipitation all year round (Cfb), and boreal with warm or cold summers and precipitation all year round (Dfb, Dfc). Almost 97% of all known I. trianguliceps locations are related to these climates. Thus, I. trianguliceps prefers climates with warm or cold summers without dry periods. Cold winters do not limit the distribution of this nidicolous tick species, which has been recorded in the European Alps and the Caucasus Mountains up to altitudes of 2400 m. Conversely, I. trianguliceps does not occur in the Mediterranean area with its hot and dry summers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ixodes trianguliceps Birula, the Eurasian shrew and vole tick (Acari, Ixodidae), is a proven vector of Babesia microti (Young 1970; Hussein 1980; Randolph 1995). It is a three-host tick species of the subgenus Exopalpiger Schulze, endemic in wide areas of Europe and Asia (Filippova 2010). In many countries such as the United Kingdom (Cotton and Watts 1967), France (Morel 1965) and Russia (Sapegina 1967), I. trianguliceps has been investigated for a long time. An early global distribution map with 45 tick locations was already presented by O’Donnell (1973). Figure 1 depicts the geographical distribution of the tick species as published by Kolonin (2009). It has been the most complete distribution map for I. trianguliceps presented so far, showing a main range of 9\(^\circ\) W–88\(^\circ\) E and an isolated occurrence at 105\(^\circ\) E. The often quoted online tick atlas of Kolonin (2009) is unfortunately no longer available, so reference is made here also to the earlier map by Kolonin (1981). Other distribution maps are restricted to national territories such as the I. trianguliceps maps of the former Soviet Union (Korenberg and Lebedeva 1969), Switzerland (Graf et al. 1979), former Yugoslavia (Tovornik 1988), as well as Great Britain and Ireland (Martyn 1988). More recent maps, which also take historical findings into account, have been compiled for Germany (Rubel et al. 2021, 2023) and Austria (Rubel and Brugger 2022). A list of all countries with I. trianguliceps reports was very recently compiled by Guglielmone et al. (2023).
All postembryonic life stages of I. trianguliceps infest mainly burrowing small mammals. It colonizes moist (not wet) habitats in deciduous, mixed and coniferous forests. In Russia, most I. trianguliceps findings are located within the dark-coniferous forest of the Central and Southern Taiga. It is less often found in pine, broad-leaved and aspen-birch forests. Occasionally, the tick penetrates into the Northern Taiga and the forest-steppe (Korenberg and Lebedeva 1969). Ixodes trianguliceps also occurs at high altitudes above the treeline. In the European Alps (Aeschlimann et al. 1970; Mahnert 1971) and the Caucasus Mountains (Filippova and Stekolnikov 2007) the tick has been found up to an altitude of 2400 m. The tick was also found in the high mountain areas of Sweden and Norway, where the northernmost location is documented north of the Arctic Circle on the Lofoten Islands (Nilsson 1974). These occurrences indicate that I. trianguliceps is a rather cold-resistant Ixodes species, but it must be emphasized that its typical off-host habitat in the soil seems to be well protected from bad frost. Only two other tick species, namely the seabird tick Ixodes uriae (Munoz-Leal and González-Acuna 2015) and the castor bean tick Ixodes ricinus have been recorded at these northern latitudes of Scandinavia.
Research into the biology of I. trianguliceps began in the 1950s and early 1960s (Vysotskaya 1951; Nikitina 1960; Lachmajer 1962). It is a nidicolous tick, i.e., living in small mammals’ dens and burrows, where it might have rather easy access to its hosts. It has mainly been found on Apodemus sylvaticus mice and Myodes glareolus voles in the United Kingdom (Bown et al. 2003), on Sorex araneus, Sorex alpinus, Sorex minutus shrews, Apodemus flavicollis mice, as well as on Microtus agrestis and Microtus nivalis voles in Austria (Mahnert 1971). Some other small mammals such as the European dormouse Glis glis and the European mole Talpa europaea (Tovornik 1988) have also been mentioned as host species. The 53 host species in Russia listed by Korenberg and Lebedeva (1969) aditionally include less common hosts such as the red fox Vulpes vulpes, ground-feeding birds such as the mistle thrush Turdus viscivorus and reptiles such as the common lizard Lacerta vivipara.
It should be noted that the global distribution of I. trianguliceps corresponds quite well to that of one of its most important hosts, the bank vole Myodes glareolus (formerly Clethrionomys glareolus), whose distribution area can be retrieved from the International Union for Conservation of Nature (2022). Ixodes trianguliceps is also considered a rare, accidental parasite of humans (Pfäffle et al. 2017). Although all life stages of I. trianguliceps appear to be active, i.e., feeding on hosts, throughout the year, there is a distinct annual cycle (Cotton and Watts 1967; Mahnert 1971; Ulmanen 1972) with most adult ticks being found from April to May. The highest activity of the nymphs was mostly observed from June to August. Larvae have a bimodal activity with a first peak in spring and a second peak in autumn. However, the seasonal activity of I. trianguliceps is subject to strong variation depending on both the climatic region and the weather of the respective year. For example, the activity peaks of all tick stages in the warm southwest of France (Gilot et al. 1976a) occur much earlier than in the higher altitudes of the European Alps (Mahnert 1971) or the higher latitudes of Scandinavia (Ulmanen 1972).
Digital world maps (Kottek et al. 2006; Rubel and Kottek 2010) and high-resolution maps for the European Alps (Rubel et al. 2017) of the Köppen–Geiger climate classification were used here to investigate the climate adaptation of I. trianguliceps. This most widespread climate classification goes back to a cooperation between the German–Russian meteorologist Wladimir Köppen and the German climatologist Rudolf Geiger (Köppen 1936; Geiger 1961). Global maps of the Köppen–Geiger climate classification have been used to characterize the suitable climate for Ixodes scapularis (Feria-Arroyo et al. 2014), Argas miniatus and Argas persicus (Muñoz-Leal et al. 2018), Haemaphysalis concinna (Rubel et al. 2018), as well as Dermacentor reticulatus and Dermacentor silvarum (Rubel et al. 2020).
In this paper new maps depicting the complete geographical distribution of I. trianguliceps as it is known to date are presented, to relate georeferenced tick sampling sites to a global climate classification.
Materials and methods
Since there was no data set on the global distribution of I. trianguliceps in Eurasia, a comprehensive literature search was carried out. For this purpose, the authors have used their personal literature collection, which has been built up over decades. It contains historical works going back to 1854, mostly in German, English, French, Italian, Spanish and Russian, and has been regularly updated with new publications via PubMed, Scopus and Google Search. This data set on the distribution of ticks in Europe and the adjacent areas of Asia and Africa was therefore not created through a systematic review specifically for this paper, but in the classic way through many years of expert work. It refers mainly to that kind of literature in which georeferenced findings are documented. For example, geographical coordinates of I. trianguliceps locations are already available for Austria (Rubel and Brugger 2022), Belgium (Obsomer et al. 2013) and Germany (Rubel et al. 2014, 2021, 2023). Digital coordinates for Great Britain and Ireland (Martyn 1988) were archived by the National Biodiversity Network (2022), and a collection of Swiss locations (Graf et al. 1979) can be obtained from the Centre Suisse de Cartographie de la Faune (2022). However, the Swiss locations were taken from the original publication. In order to close data gaps, however, tick findings were also digitized if sufficient text information on the locations or printed maps were available. According to Table 1 the following numbers of I. trianguliceps locations were incorporated: 3 in Armenia, 12 in Austria, 8 in Belgium, 8 in Bulgaria, 5 in the Czech Republic, 16 in Croatia, 5 in Estonia, 22 in Finland, 94 in France, 46 in Germany, 290 in Great Britain and Ireland, 3 in Hungary, 8 in Italy, 4 in Lithuania, 2 in the Netherlands, 6 in Norway, 15 in Poland, 4 in Romania, 52 in Russia, 73 in the Scandinavian countries, 4 in Serbia, 5 in Slovakia, 261 in the former Soviet Union, 8 in Spain, 5 in Sweden, 73 in Switzerland, 2 in Turkey, 7 in Ukraine, and 116 in former Yugoslavia.
As depicted in Table 1, most references considered describe observations from the period 1960–2000. Although there are also many publications after the year 2000, they only contain a few I. trianguliceps findings. Despite this, much effort has been expended to map these recent I. trianguliceps findings as they may confirm older occurrences. Importantly, large parts of Eurasia are not adequately covered by available studies. Thus, the handdrawn map by Korenberg and Lebedeva (1969) was digitized, without which a good coverage of the countries of the former Soviet Union would not have been possible. The same applies to the Balkans, for which the tick locations from former Yugoslavia (Tovornik 1988) were digitized. The location on the Crimea was taken from the map by O’Donnell (1973).
Digitized locations, of course, are generally of lower accuracy than locations described by geographical coordinates determined by GPS in the field. To provide evidence of this, accuracy measures were given for all data referenced in Table 1 in accordance with a scheme established in previous studies. It is distinguished between high (h \(\approx \) 0.1 km), medium (m \(\approx \) 1 km), low (l \(\approx \) 10 km) and unspecified (u) accuracies. The latter has been applied here only to the German (Rubel et al. 2014, 2021, 2023) and Austrian (Rubel and Brugger 2022) records that contain tick locations of all accuracy levels.
To visualize the geographical distribution of I. trianguliceps, the georeferenced locations were plotted on terrain maps (OpenStreetMap contributors 2017). They show the distribution patterns of the tick determined by continental-scale mountain ranges like the Himalayas and surrounding steppes and deserts. The latter were also depicted in a second type of maps, where the tick locations were plotted on climate maps. Therefore, updated global maps of the Köppen–Geiger climate classification (Rubel and Kottek 2010) were used. Generally, the Köppen–Geiger climate classification is based on 31 climate classes described by a three-letter code. The first letter distinguishes between different types of vegetation of the equatorial zone (A), the arid zone (B), the warm temperate zone (C), the boreal or snow zone (D), and the polar or ice zone (E). The second letter in the classification considers precipitation (e.g., Cf for warm temperate and precipitation all year round) and the third letter considers air temperature (e.g., Cfb warm temperate, precipitation all year round and warm summer).
The climate map (version December 2018) is provided on https://koeppen-geiger.vu-wien.ac.at together with the underlying digital data and an R code (R Development Core Team 2022) for reading and visualization. The gridded climate classification is available with a spatial resolution of 5 arcmin and representative for the 25-year period 1986–2010. It was calculated from downscaled, i.e., disaggregated (Rubel et al. 2017), temperature and precipitation fields as described by Kottek et al. (2006). With this dataset, each tick location can be related to a specific climate class in order to calculate a histogram. Recent applications of this so-called Köppen profile were, for example, presented by Grímsson et al. (2018) and Rubel et al. (2018, 2020).
Finally, the literature search included the occurrence of microorganisms or their DNA/RNA in I. trianguliceps ticks and also research on the vector competence of I. trianguliceps for any pathogens. Because this tick species is endophilous and it is usually not possible to collect its unfed stages by flagging, infections of I. trianguliceps with microorganisms have been detected only in individuals removed from hosts. However, this approach leaves it open whether the found microorganisms were freshly taken by that tick with the current bloodmeal or whether the unfed tick had already carried that infection. As a consequence, even a positive result leaves the critical eco-epidemiological question open whether or not I. trianguliceps is a vector of the found microorganism.
Results and discussion
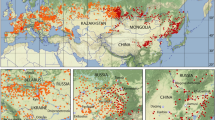
Figure 2 depicts a map of the entire distribution areas of the shrew and vole tick I. trianguliceps and a higher resolution section of the Greater Alpine Region (GAR) is shown in Fig. 3. The GAR map was chosen to demonstrate the preferred occurrence of I. trianguliceps in cooler climate regions such as the European Alps. There the tick has been found both in Switzerland at Göscheneralp (Aeschlimann et al. 1970) and in Austria at Obergurgl (Mahnert 1971) up to an altitude of 2300 m. In the Czech Republic, I. trianguliceps was found in the High Tatras near the Téry cottage at an altitude of 2016 m (Cerny 1959) and in the Russian Caucasus region at Mt. Elbrus up to an altitude of 2400 m (Filippova and Stekolnikov 2007). The tick was also found in the high mountain areas of Sweden and Norway, where the northernmost location is documented on the Lofoten Islands at 68.7\(^\circ\) N (Nilsson 1974). The southernmost location was documented in Turkey at 40.3\(^\circ\) N (Keskin and Selcuk 2021). In the Balkans, the occurrence of I. trianguliceps has also been documented down to southern latitudes of 41\(^\circ\) N (Tovornik 1988). The distribution area of I. trianguliceps is thus in the latitude belt of 40–69\(^\circ\) N. In southern Europe, this is about five degrees of latitude south of the southernmost limit shown in Fig. 1. This map adapted from Kolonin (1981, 2009) dates from before 1980, when the author apparently had no information about the occurrence of I. trianguliceps in the Balkans, in the Italian Apennines and on the western Turkish Black Sea coast. East of the Caucasus, however, the southern distribution limit is consistently ten degrees of latitude further north. The southernmost observation of I. trianguliceps in its Siberian distribution range is also the easternmost location documented near Lake Baikal at about 105\(^\circ\) E/51\(^\circ\) N (Vershinina 1988). With the westernmost I. trianguliceps findings in Ireland at 7.3\(^\circ\) W (Martyn 1988) the global distribution can be estimated. Thus, the documented distribution area extends from Ireland/Northern Spain to Western Siberia between 8\(^\circ\) W–105\(^\circ\) E and 40–69\(^\circ\) N. However, the documented locations of I. trianguliceps are unevenly distributed within this area. Clustered tick occurrences or even data gaps are mainly due to the presence or absence of regional field studies and should not be interpreted biologically.
A key result is the determination of the climate preference of I. trianguliceps crucial for its global distribution. For this purpose, the tick locations were superimposed on the Köppen–Geiger climate classification map and a frequency distribution of these tick locations in different climate zones was compiled. Figure 4 shows the climate classification map together with the Köppen profile for I. trianguliceps. The latter shows a histogram of the frequency of tick findings reported for different climate classes. Two peaks are related to the following climates: warm temperate with precipitation all year round Cf (58%) and boreal with precipitation all year round Df (41%). Thus, a total of 99% of all I. trianguliceps locations was reported in these climates, and it is evident that I. trianguliceps prefers precipitation all year round. This agrees surprisingly well with the Köppen profile for Dermacentor reticulatus (Rubel et al. 2020), a tick species with which I. trianguliceps is sympatric in large parts of its range. Due to its nidicolous off-host life, however, I. trianguliceps is even better ecologically adapted to cold, which is why it also occurs at higher altitudes and at higher geographical latitudes than D. reticulatus. The below-ground microclimate in the host burrows is not identical with that above ground. This is important to bear in mind when talking about climate adaptation in the following. Macroclimatic temperature extremes are attenuated below-ground (and also below snow in the winter).
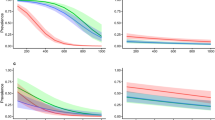
The two high alpine I. trianguliceps findings near Göscheneralp and Obergurgl described above are located in the so-called Alpine belt above the tree and forest line. This altitudinal belt—for details see Rubel et al. (2017)—is characterized by the tundra climate ET, whose lower limit is defined by the 10 \(^\circ\)C isotherm. This means that the maximum monthly mean temperature is below 10 \(^\circ\)C, such as in Obergurgl with a July mean temperature of 6.9 \(^\circ\)C (Fig. 5). As at all other I. trianguliceps locations, precipitation falls in Obergurgl all year round with an annual precipitation of 979 mm/year. During the winter months, precipitation falls as snow, resulting in about 130 days of snow cover per year (Koch et al. 2020). The mean annual temperature is negative at −1.2 \(^\circ\)C. However, it can be assumed that I. trianguliceps ticks tolerate even more extreme macroclimatic conditions than those shown in the climate diagram (Fig. 5), since the study area of Mahnert (1971) was 400 m above Obergurgl. There, the snow cover is present for about 150 days a year. A second climate diagram from Lyon, France, shows the significantly warmer Cfb climate, in which 52% of the I. trianguliceps findings collected here are located (Fig. 5). In Lyon, a mean annual temperature of 11.9 \(^\circ\)C and a mean annual precipitation of 870 mm/year were observed in the period 1986–2010. Generally, the warm temperate Cfb climate is defined for a temperature range of \(-\,3 \,^{\circ }\)C \(< T_{min} < + 18^{\circ }\) C, a maximal monthly temperature of \(T_ {max} < 22\,^{\circ }\)C, and at least four months with \(T_{mon} \ge 10\,^{\circ }\)C (Kottek et al. 2006).
Less than one percent of the I. trianguliceps findings are just outside the climate classes discussed. This can be caused by imprecise georeferenced tick findings, insufficient spatial resolution of the climate data or a temporal discrepancy between the tick findings and the climate data. Thus, the southern distribution in Europe is limited by the Mediterranean climate, characterized by the summer-dry climates Csa and Csb. Ixodes trianguliceps definitely does not occur in the Mediterranean region (light green in Fig. 4). In southern Siberia, the hot summers of the Dfa climate, which is replaced further south by the steppe climate BSk, apparently limit the spread of I. trianguliceps. In the east, the distribution of I. trianguliceps is limited by the winter-dry climates Dwb and Dwc, where the cold can enter the soil much more easily without a buffering snow cover. If one considers the shift in climate zones observed since 1900 and projected up to the year 2100 (Rubel and Kottek 2010), the distribution area of I. trianguliceps might have changed only insignificantly in the past and only small changes are to be expected for the future. There could potentially be population declines in northern France and the Balkans if those regions do indeed get warmer and drier summers. However, it must be considered that although current climate models can predict the temperature well, changes in the precipitation regime are subject to great uncertainty.
At this point it should be noted that all I. trianguliceps findings described in the literature were subjected to a plausibility check in the present study. As a result, a total of four locations in Iran (Hamidi and Bueno-Mar 2021) has been excluded from the data set, as also practiced by Guglielmone et al. (2023). These findings, with mean coordinate of 59\(^\circ\) E/36\(^\circ\) N, are far south of the known distribution area described above. In addition, Hamidi and Bueno-Mar (2021) state that I. trianguliceps was collected only from the Persian jird Meriones persicus, which inhabits dry, rocky slopes with sparse vegetation and steppe. In fact, two of the locations are in the Mediterranean climate Csa and two in the steppe climate BSk. It can thus be assumed that the I. trianguliceps ticks reported from Iran were misidentifications. In contrast, ticks collected from migratory birds in eastern Poland were included, since they occur in the natural range of I. trianguliceps. At the Kaliszany Ornithological Station, I. trianguliceps were collected from blackcaps Sylvia atricapilla and song thrushes Turdus philomelos (Zajac et al. 2022).
Table 2 provides a summary of the pathogens found in I. trianguliceps removed from hosts. A possible role of I. trianguliceps in natural foci of tick-borne encephalitis (TBE) and hemorrhagic fever renal syndrome (HFRS), which motivated the early work of Korenberg and Lebedeva (1969), was not confirmed. There is currently no study that found TBE virus in I. trianguliceps, and it is now known that HFRS is caused by hanta viruses, which are transmitted through aerosolized excrement of rodents. However, numerous pathogenic bacteria and protozoa have been found in I. trianguliceps. It must be pointed out that the finding of any tick-borne pathogens in ticks removed from hosts is no proof of vector competence. Without proven capability of transmission the vector function of a given tick species for a given pathogen is not substantiated (Kahl et al. 2002). Transmission studies with I. trianguliceps are only available for Babesia microti, which were carried out for the first time by Young (1970) in the United Kingdom. Based on this, Randolph (1995) quantified the parameters of the natural transmission cycle of B. microti between the tick vector I. trianguliceps and the host M. glareolus. In experimental infection studies, transovarial transmission of B. microti could be ruled out and the natural transmission cycle explained by host-to-vector, vector-to-vector, and vector-to-host transmission. Without experimental transmission carried out, but indicated by extensive field studies, I. trianguliceps is a putative vector for Anaplasma phagocytophilum (Bown et al. 2003, 2008). The same applies to the repeated finding of Borrelia burgdorferi s.l. spirochaetes in I. trianguliceps removed from hosts. They would also justify experimental transmission studies, but for the time being this tick species can only be called a possible vector of B. burgdorferi s.l. (Eisen 2020).
Conclusions and outlook
To summarize the current knowledge of the distribution of I. trianguliceps, a dataset of 1161 locations was collated to compile a geographical map covering its whole distribution range from Ireland and the Spanish Atlantic coast in the west to Lake Baikal in the east. Although there are numerous recent studies on I. trianguliceps (Keskin and Selcuk 2021; Mysterud et al. 2015; Obert et al. 2015; Tretyakov 2017), field studies are totally lacking in some regions or were carried out many decades ago, so that there is also potential for improving the map presented here. As already demonstrated for other tick species (Rubel et al. 2018, 2020), all known locations of I. trianguliceps were assigned climate classes using digital data from the global Köppen–Geiger climate classification (Kottek et al. 2006). The result is a very clear climate profile for I. trianguliceps, according to which the tick species occurs primarily in the warm temperate and boreal climate zones with precipitation in all seasons. However, I. trianguliceps has also occasionally been found in Alpine tundra climates. Looking at the list of pathogens found in feeding I. trianguliceps ticks (Table 2) and the only experimental transmission studies concerning B. microti, it becomes clear that there is a great need for having more such studies to prove or disprove its vector competence for some tick-borne pathogens (Bonnet and Nadal 2021) in order to support public health authorities.
The here presented data might be a suitable basis for compiling maps based on species distribution models as already applied for Dermacentor reticulatus (Brugger and Rubel 2023). The latter are part of a set of digital tick maps projected onto the virtual globe Google Earth. These Google Earth maps are currently being developed in order to offer scientists from various disciplines, but also the interested public, simple and modern access to tick distribution maps. The first maps of the distribution of I. trianguliceps have already been compiled and can be downloaded as a preview from the following link https://epidemic-modeling.vetmeduni.ac.at/.
Global geographical distribution of Ixodes trianguliceps (red area, 214), adapted and coloured from the no longer available online tick atlas by Kolonin (2009). Contour lines and dots provided with different numbers indicate the distribution of other tick species not used here
Findings of Ixodes trianguliceps superimposed on the map of the Köppen-Geiger climate classification (defined by a three-letter code and representative for the period 1986–2010) and frequency distribution of I. trianguliceps occurrence. Absolute frequencies depict the number of tick locations, relative frequencies the fraction of tick locations in each climate class. Highest frequencies of I. trianguliceps occurrence were observed in warm temperate climates with precipitation all year round (Cfb) and boreal (continental) climates with precipitation all year round (Dfb, Dfc), both with warm or cold summers
Climate diagrams for the period 1986–2010. Warm temperate climate with year-round precipitation and warm summers (Cfb) in Lyon, France, typical of more than 50% of the recorded Ixodes trianguliceps locations, and Alpine tundra climate (ET) in Obergurgl, Austria, reflecting the macroclimatic cold adaptation of I. trianguliceps at 1920 m altitude
References
Aeschlimann A, Büttiker W, Diehl PA, Eichenberger G, Immler R, Weiss N (1970) Présence d’ Ixodes trianguliceps (Birula, 1895) et d’ Ixodes apronophorus (Schulze, 1924) en Suisse (Ixodoidea; Ixodidae) (in French). Rev Suisse Zool 11:527–536
Balashov YS, Bochkov AV, Vashchonok VS, Tretyakov KA (2003) Structure and seasonal dynamics of ectoparasite community on the common shrew Sorex araneus in the Ilmen-Volkhov lowland (in Russian). Parazitologiya 37:441–454
Baráková I, Derdáková M, Selyemováb D, Chvostáč M, Špitalská E, Rosso F, Collini M, Rosá R, Tagliapietra V, Girardi M, Ramponi C, Hauffe HC, Rizzoli A (2018) Tick-borne pathogens and their reservoir hosts in Northern Italy. Ticks Tick Borne Dis 9:164–170. https://doi.org/10.1016/j.ttbdis.2017.08.012
Bespyatova LA, Bugmyrin SV, Kutenkov SA, Nikonorova IA (2019) The abundance ixodid ticks (Acari: Ixodidae) on small mammals in forest biotopes of the middle Taiga subzone of Karelia (in Russian). Parazitologiya 53:463–473. https://doi.org/10.1134/S0031184719060036
Blanarová L, Stanko M, Carpi G, Miklisová D, Vichová B, Mosansky L, Bona M, Derdáková M (2014) Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in Central Europe. Ticks Tick Borne Dis 5:928–938. https://doi.org/10.1016/j.ttbdis.2014.07.012
Blanarová L, Stanko M, Miklisová D, Vichová B, Mosansky L, Kraljik J, Bona M, Derdáková M (2016) Presence of Candidatus Neoehrlichia mikurensis and Babesia microti in rodents and two tick species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick Borne Dis 7:319–326. https://doi.org/10.1016/j.ttbdis.2015.11.008
Bonnet SI, Nadal C (2021) Experimental infection of ticks: an essential tool for the analysis of Babesia species biology and transmission. Pathogens 10:1403. https://doi.org/10.3390/pathogens10111403
Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH (2003) Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis 9:63–70. https://doi.org/10.3201/eid0901.020169
Bown KJ, Begon M, Bennett M, Birtles RJ, Burthe S, Lambin X, Telfer S, Woldehiwet Z, Ogden NH (2006) Sympatric Ixodes trianguliceps and Ixodes ricinus ticks feeding on field voles (Microtus agrestis): potential for increased risk of Anaplasma phagocytophilum in the United Kingdom? Vector Borne Zoonotic Dis 6:404–410. https://doi.org/10.1089/vbz.2006.6.404
Bown KJ, Lambin X, Telford GR, Ogden NH, Telfer S, Woldehiwet Z, Birtles RJ (2008) Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl Environ Microbiol 74:7118–7125. https://doi.org/10.1128/AEM.00625-08
Boyard C, Vourc’h G, Barnouin J (2008) The relationships between Ixodes ricinus and small mammal species at the woodland-pasture interface. Exp Appl Acarol 44:61–76. https://doi.org/10.1007/s10493-008-9132-3
Brugger K, Rubel F (2023) Tick maps on the virtual globe: first results using the example of Dermacentor reticulatus. Ticks Tick Borne Dis 14:102102. https://doi.org/10.1016/j.ttbdis.2022.102102
Cayol C, Jääskeläinen A, Koskela E, Kyröläinen S, Mappes T, Siukkola A, Kallio ER (2018) Sympatric Ixodes-tick species: pattern of distribution and pathogen transmission within wild rodent populations. Sci Rep 8:16660. https://doi.org/10.1038/s41598-018-35031-0
Centre Suisse de Cartographie de la Faune (2022) Tick maps. http://lepus.unine.ch/carto/. Accessed 20 Sept 2022
Cerny V (1959) Die Zecken (Ixodoidea) des Riesengebirges und eine Bemerkung zur systematischen Stellung der Art Exopalpiger heroldi Schulze (in German). Acta Mus Nat Prag B 51:156–160
Coipan EC, Vladimirescu AF, Ciolpan O, Teodorescu I (2011) Tick species (Acari: Ixodoidea) distribution, seasonality and host associations in Romania. Travaux du Mus Nat d’Hist Nat Grigore Antipa 54:301–317
Cotton MJ, Watts CHS (1967) The ecology of the tick Ixodes trianguliceps Birula (Arachnida; Acarina; Ixodoidea). Parasitology 57:525–531. https://doi.org/10.1017/s0031182000072401
De Pelsmaeker N, Korslund L, Steifetten O (2021) High-elevational occurrence of two tick species, Ixodes ricinus and I. trianguliceps, at their northern distribution range. Parasit Vectors 14:161. https://doi.org/10.1186/s13071-021-04604-w
Dilbaryan KP, Hovhannisyan VS (2016) Arthropods (Arthropoda) of Armenia of medical importance, their biological and ecological peculiarities. Med Sci Armenia 56:81–89. https://doi.org/10.54503/0514-7484
Doby JM, Bigaignon G, Launay H, Costil C, Lorvellec O (1990) Observation of Borrelia burgdorferi, agent of tick spirochetosis, in Ixodes trianguliceps Birula, 1895 and Ixodes acuminatus Neumann, 1901 and in Ctenophthalmus baeticus arvernus Jordan, 1931 and Megabothris turbidus, ectoparasites of forest micromammals in the western part of France (in French). Bull la Soc Francaise de Parasitol 8:311–322
Dominguez G (2014) North Spain (Burgos) wild mammals ectoparasites. Parasite 11:267–272. https://doi.org/10.1051/parasite/2004113267
Eichenberger RM, Deplazes P, Mathis A (2015) Ticks on dogs and cats: a pet owner-based survey in a rural town in Northeastern Switzerland. Ticks Tick Borne Dis 6:267–271. https://doi.org/10.1016/j.ttbdis.2015.01.007
Eisen L (2020) Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: a review. Ticks Tick Borne Dis 11:101359. https://doi.org/10.1016/j.ttbdis.2019.101359
Feria-Arroyo T, Castro-Arellano I, Gordillo-Perez G, Cavazos A, Vargas-Sandoval M, Grover A, Torres J, Medina R, de León A, Esteve-Gassent M (2014) Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasit Vectors 7:199. https://doi.org/10.1186/1756-3305-7-199
Filippova NA (2010) Uncommon zoogeographical connections in the subgenus Exopalpiger Schulze of the genus Ixodes Latreille (Acari, Ixodidae). Entomol Rev 90:793–797. https://doi.org/10.1134/S0013873810060151
Filippova NA, Stekolnikov AA (2007) Materials on the preimaginal stages of the ticks collected from small mammals in western and northern Caucasus (Acari: Ixodidae) (in Russian). Parazitologiya 41:3–22
Flavioni A (2016) Relazioni ecologiche tra roditori selvatici e parassiti ixodidi in alcune aree boschive dell'Abruzzo (in Italian), Master thesis. Univ. of Rome
Geiger R (1961) Revised new edition of Geiger, R.: Köppen-Geiger/Climate of the Earth (wall map 1:16 M, in German). Klett-Perthes, Gotha
Gilot B, Beaucournu JC, Pautou G, Fayard A, Moncada E (1976a) Distribution and ecology of Ixodes trianguliceps (Birula, 1895) (Acarina, Ixodoidea) in France, particularly in the South-East (in French). Acta Trop 33:254–286
Gilot B, Moncada E, Pautou G (1976b) Presence in France of Ixodes apronophorus (Schulze, 1924). Ixodoidea, Ixodidae (in French). Ann Parasitol Hum Comp 51:601–603. https://doi.org/10.1051/parasite/1976515601
Gilot B, Pautou G, Gosalbez J, Moncada E (1976c) Contribution to study of Ixodidae (Acarina, Ixodoidea) in Cantabric Mountains (Spain) (in French). Ann Parasitol Hum Comp 51:241–254. https://doi.org/10.1051/parasite/1976512241
Gilot B, Pautou G, Moncada E, Lachet B, Christin JG (1979) La cartographie des populations de tiques exophiles par le biais de la vegetation: bases écologiques, interet epidemiologique (in French). Doc de Cart Ecol 22:65–80
Graf JF, Mermod C, Aeschlimann A (1979) New data on the distribution ecology and biology of Ixodes trianguliceps in Switzerland (Ixodoidea, Ixodidae) (in French). Bull Soc Neuchâtel Sci Nat 102:55–68
Grandi G, Chiappa G, Ullman K, Lindgren P, Olivieri E, Sassera D, Östlund E, Omazic A, Perissinotto D, Söderlund R (2023) Characterization of the bacterial microbiome of Swedish ticks through 16S rRNA amplicon sequencing of whole ticks and of individual tick organs. Parasit Vectors 16:39. https://doi.org/10.1186/s13071-022-05638-4
Grigoryeva LA, Tretyakov KA (1998) Peculiarity of the parasitic system ixodid ticks-borrelia-micromammalia in the north-west of Russia. Parazitologiya 32:422–430
Grímsson F, Grimm G, Potts A, Zetter R, Renner SS (2018) A Winteraceae pollen tetrad from the early Paleocene of Western Greenland, and the fossil record of Winteraceae in Laurasia and Gondwana. J Biogeogr 45:567–581
Guglielmone AA, Nava S, Robbins RG (2023) Geographic distribution of the hard ticks (Acari: Ixodida: Ixodidae) of the world by countries and territories. Zootaxa 5251(1):1–274. https://doi.org/10.11646/zootaxa.5251.1.1
Guryčová D (1998) First isolation of Francisella tularensis subsp. tularensis in Europe. Eur J Epidemiol 14:797–802. https://doi.org/10.1023/A:1007537405242
Haitlinger R (2010) Arthropods (Acari, Anoplura, Siphonaptera) of small mammals of Lubelskie province. Zesz. Nauk. UP Wroc., Biol. Hod. Zwierz LXI 579:21–48
Hamidi K, Bueno-Mar R (2021) Host-ectoparasite associations; the role of host traits, season and habitat on parasitism interactions of the rodents of northeastern Iran. J Asia-Pac Entomol 24:308–319. https://doi.org/10.1016/j.aspen.2020.12.009
Henkel G, Centurier C, Weiland G (1983) Isolation of a rodent babesiasis in southern Germany and its characterization (in German). Berl Münch Tierärztl Wochenschr 96:242–244
Hubálek Z, Juricová Z, Halouzka J (1990) Francisella tularensis from ixodid ticks in Czechoslovakia. Folia Parasitol 37:255–260
Hussein HS (1980) Ixodes trianguliceps: seasonal abundance and role in the epidemiology of Babesia microti infection in north-western England. Ann Trop Med Parasitol 74:531–539. https://doi.org/10.1080/00034983.1980.11687381
Hvidsten D, Frafjord K, Gray JS, Henningsson AJ, Jenkins A, Kristiansen BE, Lager M, Rognerud B, Slatsve AM, Stordal F, Stuen S, Wilhelmsson P (2020) The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis 11:101388. https://doi.org/10.1016/j.ttbdis.2020.101388
Igolkina YP, Rar VA, Yakimenko VV, Malkova MG, Tancev AK, Tikunov AY, Epikhina TI, Tikunova NV (2015) Genetic variability of Rickettsia spp. in Ixodes persulcatus/Ixodes trianguliceps sympatric areas from Western Siberia, Russia: Identification of a new Candidatus Rickettsia species. Infect Genet Evol 34:88–93. https://doi.org/10.1016/j.meegid.2015.07.015
International Union for Conservation of Nature (2022) IUCN red list of threatened species. https://www.iucnredlist.org Accessed 7 Oct 2022
Izdebska JN, Kadulski S (2011) New records of the tick Ixodes trianguliceps Birula, 1895 in the Kashubian Coastland, Żuławy Wiślane and Tuchola Forest. In: Buczek A, Błaszak C (eds) Arthropods. Human and animal parasites. Akapit, Berlin
Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, Heyman P, van Maanen C, Butler CM, Földvári G, Szekeres S, van Duijvendijk G, Tack W, Rijks JM, van der Giessen J, Takken W, van Wieren SE, Takumi K, Sprong H (2014) Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors 7:365. https://doi.org/10.1186/1756-3305-7-365
Janisch M (1959) A hazai kullancs fauna feltérképezése (in Hungarian). Allattani Közlemények 47:103–110
Kahl O, Gern L, Eisen L, Lane R (2002) Ecological research on Borrelia burgdorferi sensu lato: terminology and some methodological pitfalls. In: Gray JS, Kahl O, Lane RS, Stanek G (eds) Lyme borreliosis: biology, epidemiology and control. CABI Publishing, New York, pp 29–46
Kerbabaev EB, Tsushba AC (2011) Ixodofauna in Abkhazia and the adjacent region (in Russian). Russ J Parasitol 1:18–26
Keskin A, Selcuk AY (2021) A survey for tick (Acari: Ixodidae) infestation on some wild mammals and the first record of Ixodes trianguliceps Birula in Turkey. Syst Appl Acarol 26:2209–2220
Koch R, Gobiet A, Olefs M (2020) Study on past and future snow cover development in the Obergurgl ski area (in German), Zentralanstalt für Meteorologie und Geodynamik (ZAMG). https://fuse-at.ccca.ac.at. Accessed 23 Sept 2022
Kolchanova LP, Bragina EA (2011) Detection of Ehrlichia and Anaplasma DNA in Ixodes (Exopalpiger) trianguliceps Bir. ticks in Tyumen Province. Entomol Rev 91:1181–1183. https://doi.org/10.1134/S0013873811090119
Kolonin GV (1981) World distribution of ixodid ticks (genus Ixodes). Nauka, Moscow
Kolonin GV (2009) Fauna of Ixodid Ticks of the World (Acari, Ixodidae). http://www.kolonin.org. Accessed 10 June 2014
Köppen W (1936) Das geographische System der Klimate (The geographic system of climates). In: Köppen W, Geiger R (eds) Handbuch der Klimatologie. Teil C. Borntraeger, Berlin
Korenberg EI, Lebedeva NN (1969) Distribution and some general features of the ecology of Ixodes trianguliceps Bir. in the Soviet Union. Folia Parasitol 16:143–152
Korenberg EI, Kovalevskii YV, Gorelova NB, Nefedova VV (2015) Comparative analysis of the roles of Ixodes persulcatus and I. trianguliceps ticks in natural foci of ixodid tick-borne borrelioses in the Middle Urals, Russia. Ticks Tick Borne Dis 6:316–321. https://doi.org/10.1016/j.ttbdis.2015.02.004
Kormilitsyna MI, Korenberg EI, Kovalevskii YV, Meshcheryakova IS (2016) The first molecular genetic identification of the tularemia pathogen in Ixodes trianguliceps Bir. ticks in Russia. Mol Gen Microbiol Virol 31:82–86
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen–Geiger climate classification updated. Meteorol Z 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Krčmar S (2012) Hard ticks (Acari, Ixodidae) of Croatia. ZooKeys 234:19–57
Lachmajer J (1962) The ecology of the tick Ixodes trianguliceps Bir., 1895. Bull lnst Mar Med Gdansk 13:149–160
L’Hostis M, Dumon H, Fusade A, Lazareff S, Gorenflot A (1996) Seasonal incidence of Ixodes ricinus ticks (Acari: Ixodidae) on rodents in western France. Exp Appl Acarol 20:359–368. https://doi.org/10.1007/BF00130548
Lutta AS (1968) Ixodes trianguliceps Bir. and its distribution in Karelia (in Russian). Parazitologiya 2:142–147
Mahnert V (1971) Parasitologische Untersuchungen an alpinen Kleinsäugern: Ixodoidea (Acari) (in German). Mitt Schweiz Entomol Ges 44:323–332. https://doi.org/10.5169/seals-401663
Manilla G (1990) Nuove osservazioni faunistiche e biologiche sulle zecche d’Italia (Acari: Ixodoidea) (Nota V) (in Italian). Atti della Soc Ital Scienze Nat del Museo Civico di Storia Nat Milano 131:433–441
Martello E, Mannelli A, Grego E, Ceballos LA, Ragagli C, Stella MC, Tomassone L (2019) Borrelia burgdorferi sensu lato and spotted fever group rickettsiae in small rodents and attached ticks in the Northern Apennines, Italy. Ticks Tick Borne Dis 10:862–867. https://doi.org/10.1016/j.ttbdis.2019.04.005
Martyn KP (1988) Provisional atlas of the ticks (Ixodidea) of the British Isles. Biological Record Centre, Abbots Ripton, p 62
Mihalca AD, Dumitrache MO, Magdas C, Gherman CM, Domsa C, Mircean V, Ghira IV, Pocora V, Ionescu DT, Barabási SS, Cozma V, Sándor AD (2012) Synopsis of the hard ticks (Acari: Ixodidae) of Romania with update on host associations and geographical distribution. Exp Appl Acarol 58:183–206. https://doi.org/10.1007/s10493-012-9566-5
Morel PC (1965) Présence en France de Exopalpiger trianguliceps (Birula, 1895) (Acariens, Ixodoidea) (in French). Ann Parasitol Hum Comp 40:240–242
Morini E, Pisanu B, Zozzoli R, Solano E, Olivieri E, Sassera D, Montagna M (2018) Arthropods and associated pathogens from native and introduced rodents in Northeastern Italy. Parasitol Res 117:3237–3243. https://doi.org/10.1007/s00436-018-6022-4
Munoz-Leal S, González-Acuna D (2015) The tick Ixodes uriae (Acari: Ixodidae): hosts, geographical distribution, and vector roles. Ticks Tick Borne Dis 6:843–868. https://doi.org/10.1016/j.ttbdis.2015.07.014
Muñoz-Leal S, Venzal JM, Nava S, Reyes M, Martins TF, Leite RC, Vilela VLR, Benatti HR, Ríos-Rosas D, Barros-Battesti DM, González-Acuña D, Labruna MB (2018) The geographic distribution of Argas (Persicargas) miniatus and Argas (Persicargas) persicus (Acari: Argasidae) in America, with morphological and molecular diagnoses from Brazil, Chile and Cuba. Ticks Tick Borne Dis 9:44–56
Mysterud A, Byrkjeland R, Qviller L, Viljugrein H (2015) The generalist tick Ixodes ricinus and the specialist tick Ixodes trianguliceps on shrews and rodents in a northern forest ecosystem—a role of body size even among small hosts. Parasit Vectors 8:639. https://doi.org/10.1186/s13071-015-1258-7
Naglova GI, Naglov VA (1983) Materials on the distribution and ecology of Ixodes trianguliceps Bir., 1895 (Ixodidae) in the Kharkov district (in Russian). Parazitologiya 17:409–410
National Biodiversity Network (2022) NBN Atlas occurrence. https://nbnatlas.org. Accessed 1 May 2022
Nebogatkin IV (2015) Features of parasitism of ticks on dogs in the urban landscape in megalopolis of Kyiv. Conference paper
Nikitina NA (1960) The biology of Ixodes trianguliceps. Med Parazitol 6:708
Nilsson A (1974) Distribution, host relations, and seasonal occurrence of Ixodes trianguliceps Birula (Acari) in Fennoscandia. Folia Parasitol 21:233–241
Nosek J, Lichard M, Sztankay M (1967) The ecology of ticks in the Tribec and Hronský Inovec mountains. Bull World Health Organ 36(Suppl. 1):49–59
Nowak-Chmura M (2013) The tick fauna of Central Europe (in Polish). Scientific Publisher of the Pedagogical University of Krakow, Krakow
Obert AS, Kurepina NY, Bezrukov GV, Merkushev OA, Cherkashina EN, Kalinina UV (2015) Ixodid ticks as carriers of human transmissible infectious diseases in Altai Krai (in Russian). J Altai Branch Russ Geogr Soc 37:82–89
Obiegala A, Pfeffer M, Pfister K, Karnath C, Silaghi C (2015) Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick Borne Dis 6:445–449. https://doi.org/10.1016/j.ttbdis.2015.03.005
Obsomer V, Wirtgen M, Linden A, Claerebout E, Heyman P, Heylen D, Madder M, Maris J, Lebrun M, Tack W, Lempereur L, Hance T, Van Impe G (2013) Spatial disaggregation of tick occurrence and ecology at a local scale as a preliminary step for spatial surveillance of tick-borne diseases: general framework and health implications in Belgium. Parasit Vectors 6:190. https://doi.org/10.1186/1756-3305-6-190
O’Donnell TG (1973) Density and host relations of Ixodes trianguliceps Bir. in woodland and grassland areas. In: Daniel M, Rosický B (eds) Proc 3rd Int Cong Acarology in Prague 1971. Springer, Dordrecht, pp 783–787
OpenStreetMap Contributors (2017) Planet dump. https://planet.osm.org, https://www.openstreetmap.org
Paulauskas A, Radzijevskaja J, Turcinavicien J, Ambrasien D, Galdikait E (2010) Data on some ixodid tick species (Acari, Ixodidae) in the Baltic countries. New Rare Lith Insect Species 22:43–51
Perez G, Bastian S, Chastagner A, Agoulon A, Plantard O, Vourc’h G, Butet A (2017) Ecological factors influencing small mammal infection by Anaplasma phagocytophilum and Borrelia burgdorferi s.l. in agricultural and forest landscapes. Environ Microbiol 19:4205–4219. https://doi.org/10.1111/1462-2920.13885
Petrović A, Ivanović I, Jurisić A, Bursić V, Popović A, Petrović M, Gvozdenac S (2016) Tick (Acari: Ixodidae) infestation of water and bank voles (Rodentia: Arvicolinae). In: Proc. 2nd Int. Symp. Vet. Med., Belgrade, June 22–24, 2016, pp 127–134
Pfäffle MP, Petney TN, Madder M (1895) Ixodes trianguliceps Birula, 1895. In: Estrada-Peña A, Mihalca AD, Petney TN (eds) Ticks of Europe and North Africa. A guide to species identification. Springer, Cham, pp 167–171
Pisanu B, Marsot M, Marmet J, Chapuis JL, Réale D, Vourc’h G (2010) Introduced Siberian chipmunks are more heavily infested by ixodid ticks than are native bank voles in a suburban forest in France. Int J Parasitol 40:1277–1283. https://doi.org/10.1016/j.ijpara.2010.03.012
R Development Core Team (2022) R: a language and environment for statistical computing, version 4.2.1. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Rageau J (1972) Repartition geographique et role pathogene des tiques (Acariens: Argasidae et Ixodidae) en France (in French). Wiad Parazytol 18:707–719
Randolph SE (1995) Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus). Parasitology 110:287–295. https://doi.org/10.1017/S0031182000080872
Rar V, Yakimenko V, Makenov M, Tikunov A, Epikhina T, Tancev A, Bobrova O, Tikunova N (2016) High prevalence of Babesia microti ‘Munich’ type in small mammals from an Ixodes persulcatus/Ixodes trianguliceps sympatric area in the Omsk region, Russia. Parasitol Res 115:3619–3629. https://doi.org/10.1007/s00436-016-5128-9
Rubel F, Brugger K (2022) Maps of ticks (Acari: Argasidae, Ixodidae) for Austria and South Tyrol, Italy. Exp Appl Acarol 86:211–233. https://doi.org/10.1007/s10493-022-00688-w
Rubel F, Kottek M (2010) Observed and projected climate shifts 1901–2100 depicted by world maps of the Köppen–Geiger climate classification. Meteorol Z 19:135–141. https://doi.org/10.1127/0941-2948/2010/0430
Rubel F, Brugger K, Monazahian M, Habedank B, Dautel H, Leverenz S, Kahl O (2014) The first German map of georeferenced ixodid tick locations. Parasit Vectors 7:477. https://doi.org/10.1186/s13071-014-0477-7
Rubel F, Brugger K, Haslinger K, Auer I (2017) The climate of the European Alps: shift of very high resolution Köppen–Geiger climate zones 1800–2100. Meteorol Z 26:115–125. https://doi.org/10.1127/metz/2016/0816
Rubel F, Brugger K, Walter M, Vogelgesang JR, Didyk YM, Fu S, Kahl O (2018) Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis 9:1080–1089. https://doi.org/10.1016/j.ttbdis.2018.04.002
Rubel F, Brugger K, Belova OA, Kholodilov IS, Didyk YM, Kurzrock L, García-Pérez AL, Kahl O (2020) Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp Appl Acarol 82:95–123. https://doi.org/10.1007/s10493-020-00533-y
Rubel F, Brugger K, Chitimia-Dobler L, Dautel H, Meyer-Kayser E, Kahl O (2021) Atlas of ticks (Acari: Argasidae, Ixodidae) in Germany. Exp Appl Acarol 84:183–214. https://doi.org/10.1007/s10493-021-00619-1
Rubel F, Dautel H, Nijhof AM, Kahl O (2022) Ticks in the metropolitan area of Berlin, Germany. Ticks Tick Borne Dis 13:102029. https://doi.org/10.1016/j.ttbdis.2022.102029
Rubel F, Zaenker S, Weigand A, Weber D, Chitimia-Dobler L, Kahl O (2023) Atlas of ticks (Acari: Argasidae, Ixodidae) in Germany: 1st data update. Exp Appl Acarol 89:251–274. https://doi.org/10.1007/s10493-023-00784-5
Sabitova Y, Rar V, Tikunov A, Yakimenko V, Korallo-Vinarskaya N, Livanova N, Tikunova N (2023) Detection and genetic characterization of a putative novel Borrelia genospecies in Ixodes apronophorus/Ixodes persulcatus/Ixodes trianguliceps sympatric areas in Western Siberia. Ticks Tick Borne Dis 14:102075. https://doi.org/10.1016/j.ttbdis.2022.102075
Sapegina VF (1967) Vertical distribution of Ixodes trianguliceps Bir. in the north-eastern Altai (in Russian). Parazitologiya 1:243–244
Siuda K, Stanko M, Piksa K, Górz A (2009) Ticks (Acari: Ixodida) parasitizing bats in Poland and Slovakia. Wiadomooeci Parazytol 55:39–45
Solarz K, Asman M, Cuber P, Gomółka P, Komosińska B, Nazarkiewicz M (2010) The abundance of Ixodes ricinus L. tick (Acari: Ixodida: Ixodidae) in the Zachwyt Valley (Ojców National Park) during the autumn peak of tick activity (in Polish). Pradnik Prace Muz Szafera 20:323–332
Starikov VP, Mayorova AD, Sarapultseva ES, Bernikov KA, Nakonechny NV, Morozkina AV, Borodin AV, Petukhov VA (2017) Materials on Ixodes ticks (Ixodidae) of the Khatny–Mansi autonomous territory—Yugra (in Russian). Samarskii Nauchnyi Vestnik 6:88–91. https://doi.org/10.17816/snv201762117.t18043
Svitálková Z, Harustiaková D, Mahriková L, Berthová L, Slovák M, Kocianová E, Kazimirová M (2015) Anaplasma phagocytophilum prevalence in ticks and rodents in an urban and natural habitat in South-Western Slovakia. Parasit Vectors 8:276. https://doi.org/10.1186/s13071-015-0880-8
Tovornik D (1988) Geographic distribution and other population parameters of Ixodes (Exopalpiger) trianguliceps (Birula, 1895) in Yugoslavia. Bioloski vestnik 36:33–54
Tretyakov KA (2017) Structure and seasonal dynamics of ectoparasite community on the pygmy shrew Sorex minutus in the north of Ilmen-Volkhov lowland (in Russian). Parazitologiya 51:428–435
Ulmanen I (1972) Distribution and ecology of Ixodes trianguliceps Birula (Acarina, Ixodidae) in Finland. Ann Zool Fennici 9:111–115
van Duijvendijk G, Krijger I, van Schaijk M, Fonville M, Gort G, Sprong H, Takken W (2022) Seasonal dynamics of tick burden and associated Borrelia burgdorferi s.l. and Borrelia miyamotoi infections in rodents in a Dutch forest ecosystem. Exp Appl Acarol 87:235–251. https://doi.org/10.1007/s10493-022-00720-z
Vershinina TA (1988) Ixodes trianguliceps (Parasitiformes, Ixodidae) in South Pribaikalje (in Russian). Parazitologiya 22:336
Vikentjeva M, Geller J, Remm J, Golovljova I (2021) Rickettsia spp. in rodent-attached ticks in Estonia and first evidence of spotted fever group Rickettsia species Candidatus Rickettsia uralica in Europe. Parasit Vectors 14:65. https://doi.org/10.1186/s13071-020-04564-7
Vysotskaya SO (1951) On the biology of the ixodid tick Ixodes trianguliceps Bir. Parazitol Sborn Zool lnst Akad Nauk SSSR 13:105–110
Young AS (1970) Studies on blood parasites of small mammals with special reference to piroplasms, PhD thesis, University of London
Zajac Z, Kulisz J, Kunc-Kozioł R, Wozniak A, Filipiuk M, Rudolf R, Bartosik K, Cabezas-Cruz A (2022) Tick infestation in migratory birds of the Vistula River Valley, Poland. Int J Environ Res Public Health 19:13781. https://doi.org/10.3390/ijerph192113781
Zolotov PE, Paulkina MK, Moravek KL, Buker VP, Zaharova SN, Nosova AN, Danilina LI, Pavlovskaya MA (1974) On the ecology of ixodid ticks of the Leningrad region (in Russian). Parazitologiya 8:116–122
Acknowledgements
The authors are greatful to Jasna Dolenc Koce, Editor-in-Chief of Acta Biologica Slovenica (the successor of Bioloski vestnik), for providing the paper Tovornik (1988) and to Ard M. Nijhof for providing the paper Gilot et al. (1976b). Georg Henkel sent us his doctoral thesis and a paper on the detection of Babesia in ticks and small mammals in southern Germany (Henkel et al. 1983), and Sara Hösch supported the digitization of data.
Funding
Open access funding provided by University of Veterinary Medicine Vienna.
Author information
Authors and Affiliations
Contributions
FR and OK wrote the main manuscript text and FR compiled the data and prepared the figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rubel, F., Kahl, O. The Eurasian shrew and vole tick Ixodes trianguliceps: geographical distribution, climate preference, and pathogens detected. Exp Appl Acarol 90, 47–65 (2023). https://doi.org/10.1007/s10493-023-00797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00797-0