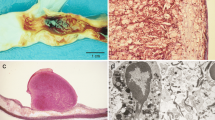
Abstract
Progression of atherosclerotic plaques into life-threatening lesions is associated with angiogenesis which contributes to intraplaque hemorrhages and plaque instability. The lack of adequate models for the study of human plaque-induced angiogenesis has limited progress in this field. We describe here a novel ex vivo model which fills this gap. Plaques obtained from 15 patients who underwent endarterectomy procedures were co-cultured in collagen gels with rat aorta rings which served as read-out of human plaque angiogenic activity. The majority of plaque fragments markedly stimulated angiogenic sprouting from the aortic rings while concurrently promoting the outgrowth of resident macrophages from the aortic adventitia. This stimulatory activity correlated with the presence of intraplaque macrophages. Proteomic analysis of plaque secretomes revealed heterogeneity of macrophage-stimulatory cytokine and angiogenic factor production by different plaques. VEGF was identified in some of the plaque secretomes. Antibody-mediated blockade of VEGF had significant but transient inhibitory effect on angiogenesis, which suggested redundancy of plaque-derived angiogenic stimuli. Pharmacologic ablation of adventitial macrophages permanently impaired the angiogenic response of aortic rings to plaque stimuli. Our results show that human plaque-induced angiogenesis can be reproduced ex vivo using rat aortic rings as read-out of plaque angiogenic activity. This model can be used to identify key cellular and molecular mechanisms responsible for the neovascularization of human plaques.






Similar content being viewed by others
References
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW et al (2011) Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123:933–944
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R (2003) Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349:2316–2325
Falke P, Matzsch T, Sternby NH, Bergqvist D, Stavenow L (1995) Intraplaque haemorrhage at carotid artery surgery—a predictor of cardiovascular mortality. J Intern Med 238:131–135
Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, Cai J, Ferguson MS, Yuan C (2007) The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology 244:64–77
Michel JB, Virmani R, Arbustini E, Pasterkamp G (2011) Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 32:1977–1985, 1985a, 1985b, 1985c
Davies MJ, Thomas AC (1985) Plaque fissuring—the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J 53:363–373
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25:2054–2061
Zhang Y, Cliff WJ, Schoefl GI, Higgins G (1993) Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol 143:164–172
Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A (2007) Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol 27:1259–1268
Jeziorska M, Woolley DE (1999) Neovascularization in early atherosclerotic lesions of human carotid arteries: its potential contribution to plaque development. Hum Pathol 30:919–925
Ho-Tin-Noe B, Michel JB (2011) Initiation of angiogenesis in atherosclerosis: smooth muscle cells as mediators of the angiogenic response to atheroma formation. Trends Cardiovasc Med 21:183–187
Hatsukami TS, Ferguson MS, Beach KW, Gordon D, Detmer P, Burns D, Alpers C, Strandness DE Jr (1997) Carotid plaque morphology and clinical events. Stroke 28:95–100
Nicosia RF (2009) The aortic ring model of angiogenesis: a quarter century of search and discovery. J Cell Mol Med 13:4113–4136
Nicosia RF, Zhu WH, Fogel E, Howson KM, Aplin AC (2005) A new ex vivo model to study venous angiogenesis and arterio-venous anastomosis formation. J Vasc Res 42:111–119
Aplin AC, Fogel E, Zorzi P, Nicosia RF (2008) The aortic ring model of angiogenesis. Methods Enzymol 443:119–136
Gelati M, Aplin AC, Fogel E, Smith KD, Nicosia RF (2008) The angiogenic response of the aorta to injury and inflammatory cytokines requires macrophages. J Immunol 181:5711–5719
Ligresti G, Aplin AC, Zorzi P, Morishita A, Nicosia RF (2011) Macrophage-derived tumor necrosis factor-alpha is an early component of the molecular cascade leading to angiogenesis in response to aortic injury. Arterioscler Thromb Vasc Biol 31:1151–1159
Schneider CA, Rasband WS, Eliceiri KW (2018) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Zhu WH, Guo X, Villaschi S, Francesco NR (2000) Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest 80:545–555
Aplin AC, Zhu WH, Fogel E, Nicosia RF (2009) Vascular regression and survival are differentially regulated by MT1-MMP and TIMPs in the aortic ring model of angiogenesis. Am J Physiol Cell Physiol 297:C471–480
Kurachi K, Davie EW, Strydom DJ, Riordan JF, Vallee BL (1985) Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry 24:5494–5499
Rosen EM, Goldberg ID (1995) Scatter factor and angiogenesis. Adv Cancer Res 67:257–279
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309
Song H, Yin D, Liu Z (2012) GDF-15 promotes angiogenesis through modulating p53/HIF-1a signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep 39:4017–4022
Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ (1996) Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271:736–741
Hu DE, Hori Y, Fan TP (1993) Interleukin-8 stimulates angiogenesis in rats. Inflammation 17:135–143
Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ (2000) Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96:34–40
Asare Y, Schmitt M, Bernhagen J (2013) The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemost 109:391–398
Liekens S, Schols D, Hatse S (2018) CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharm Des 16:3903–3920
Nicosia RF, Nicosia SV, Smith M (1994) Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol 145:1023–1029
Movahedi B, Gysemans C, Jacobs-Tulleneers-Thevissen D, Mathieu C, Pipeleers D (2008) Pancreatic duct cells in human islet cell preparations are a source of angiogenic cytokines interleukin-8 and vascular endothelial growth factor. Diabetes 57:2128–2136
Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, Zhong H, Hollingsworth RE, Thompson R, Whiteford JR, Barger AC (2015) Interleukin-6 stimulates defective angiogenesis. Cancer Res 75:3098–3107
Aplin AC, Fogel E, Nicosia RF (2010) MCP-1 promotes mural cell recruitment during angiogenesis in the aortic ring model. Angiogenesis 13:219–226
Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ (1999) Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol 154:1125–1135
Aplin AC, Gelati M, Fogel E, Carnevale E, Nicosia RF (2006) Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol Genomics 27:20–28
Martinet W, Schrijvers DM, De Meyer GR (2011) Necrotic cell death in atherosclerosis. Basic Res Cardiol 106:749–760
Parma L, Baganha F, Quax PHA, de Vries MR (2017) Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol 816:107–115
Barger AC, Beeuwkes R, Lainey LL, Silverman KJ (1984) Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med 310:175–177
Guo L, Harari E, Virmani R, Finn AV (2017) Linking hemorrhage, angiogenesis, macrophages, and iron metabolism in atherosclerotic vascular diseases. Arterioscler Thromb Vasc Biol 37:e33–e39
Li Y, Zhu Y, Deng Y, Liu Y, Mao Y, Sun J (2016) The therapeutic effect of bevacizumab on plaque neovascularization in a rabbit model of atherosclerosis during contrast-enhanced ultrasonography. Sci Rep 6:30417
Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, Gupta A, Jenkins AL, Lipinski MJ, Kim J, Chhour P et al (2018) CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest 128:1106–1124
Olson FJ, Stromberg S, Hjelmgren O, Kjelldahl J, Fagerberg B, Bergstrom GM (2011) Increased vascularization of shoulder regions of carotid atherosclerotic plaques from patients with diabetes. J Vasc Surg 54:1324–1331
Zorzi P, Aplin AC, Smith KD, Nicosia RF (2010) Technical advance: the rat aorta contains resident mononuclear phagocytes with proliferative capacity and proangiogenic properties. J Leukoc Biol 88:1051–1059
Wang J, Wang Y, Wang J, Guo X, Chan EC, Jiang F (2018) Adventitial activation in the pathogenesis of injury-induced arterial remodeling: potential implications in transplant vasculopathy. Am J Pathol 188:838–845
Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO (2009) Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 174:1097–1108
Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A, Narula J, Kolodgie FD, Virmani R (2012) Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 59:166–177
Bo WJ, Murcuri M, Tucker R, Bond MG (2018) The human carotid atherosclerotic plaque stimulates angiogenesis on the chick chorioallantoic membrane. Atherosclerosis 94:71–79
Alpern-Elran H, Morog N, Robert F, Hoover G, Kalant N, Brem S (2018) Angiogenic activity of the atherosclerotic carotid artery plaque. J Neurosurg 70:942–945
Acknowledgements
This work was supported in part by a Grant-in-aid (17GRNT33410141) from the American Heart Association and by the VA Puget Sound Health Care System. The contents of this paper do not represent the views of the U.S. Department of Veteran Affairs or the United States Government. We gratefully acknowledge the support of the Vascular Surgery Team of VA Puget Sound Health Care System for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were performed with approval from the Veterans Administration Puget Sound Health Care System Institutional Review Board and comply with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Research involving animal rights
All animal procedures were performed with approval from the Veterans Administration Puget Sound Health Care System Institutional Animal Care and Use Committee and followed National Institutes of Health Guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10456_2019_9667_MOESM1_ESM.jpg
Supplement 1 Densitometric analysis of dot blots of conditioned media obtained from human plaques and reacted for angiogenic factors and cytokines/chemokines. Plaque numbers (1, 2, 4, 5, 6, 7, 8) identify the same plaques used in the angiogenesis assay shown in Fig. 1c. Quantitative protein analysis was performed by normalizing the intensity of each analyte to internal reference controls. Note that there is significant heterogeneity in the relative levels of analytes in tested samples (see also Fig. 2) (JPEG 1931 kb)
Rights and permissions
About this article
Cite this article
Aplin, A.C., Nicosia, R.F. The plaque-aortic ring assay: a new method to study human atherosclerosis-induced angiogenesis. Angiogenesis 22, 421–431 (2019). https://doi.org/10.1007/s10456-019-09667-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-019-09667-z