Abstract
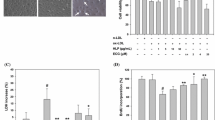
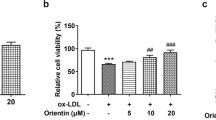
Atherosclerosis is a disease resulting from impaired endothelial function, often caused by oxidant injury or inflammation. Endothelial progenitor cells (EPCs) play a critical role in repairing damaged endothelium and protecting against atherosclerosis. Quercitrin, a plant-derived flavonoid compound, displays antioxidant and anti-inflammatory activities. In this study, we showed that quercitrin treatment reduced the apoptosis of EPCs caused by oxidized low-density lipoprotein (ox-LDL) in a dose-dependent manner. Quercitrin improved tube formation, migration and adhesion of ox-LDL-treated EPCs. To determine the effect of quercitrin in vivo, EPCs treated with or without ox-LDL and quercitrin were locally injected into the ischemic hind limb muscle of nude mice. Those injected with EPCs treated with ox-LDL and quercitrin showed significantly increased local accumulation of EPCs, blood flow recovery and capillary density compared with the control and ox-LDL only groups. Furthermore, we showed that quercitrin enhanced autophagy and upregulated mitogen-activated protein kinase and ERK phosphorylation in a dose-dependent manner in vitro. Autophagy inhibitors, chloroquine and 3-methyladenine, abrogated quercitrin-enhanced autophagy caused by ox-LDL as evidenced by decreased numbers of branch points, migratory cells and adherent cells, and increased numbers of apoptotic cells. The ERK inhibitor PD98059 abrogated quercitrin-enhanced autophagy, as identified by decreased autophagosome formation and downregulated ERK phosphorylation. The inhibition of ERK did not affect the expression of Rac1, but enhanced phosphorylation of Akt. Quercitrin treatment also increased the expression of E-cadherin, and PD98059 abrogated the upregulation of E-cadherin induced by quercitrin. Our findings suggested that autophagy is a protective mechanism in EPCs exposed to oxidative damage. Quercitrin can promote autophagy through the activation of ERK and the ERK signaling pathway is therefore thought to play a pivotal role in mediating the protective effects on EPCs.








Similar content being viewed by others
References
Aicher A, Zeiher AM, Dimmeler S (2005) Mobilizing endothelial progenitor cells. Hypertension 45(3):321–325. doi:10.1161/01.HYP.0000154789.28695.ea
Hristov M, Zernecke A, Liehn EA, Weber C (2007) Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost 98(2):274–277
Bauersachs J, Thum T (2007) Endothelial progenitor cell dysfunction: mechanisms and therapeutic approaches. Eur J Clin Investig 37(8):603–606. doi:10.1111/j.1365-2362.2007.01833.x
Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ (2004) C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109(17):2058–2067. doi:10.1161/01.CIR.0000127577.63323.24
Eizawa T, Ikeda U, Murakami Y, Matsui K, Yoshioka T, Takahashi M, Muroi K, Shimada K (2004) Decrease in circulating endothelial progenitor cells in patients with stable coronary artery disease. Heart 90(6):685–686
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89(1):E1–E7
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. New Engl J Med 353(10):999–1007. doi:10.1056/NEJMoa043814
Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H (2003) Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 93(10):980–989. doi:10.1161/01.RES.0000099245.08637.CE
Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G (2002) Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 22(10):1567–1572
Salvayre R, Auge N, Benoist H, Negre-Salvayre A (2002) Oxidized low-density lipoprotein-induced apoptosis. Biochim Biophys Acta 1585(2–3):213–221
Ding Z, Wang X, Schnackenberg L, Khaidakov M, Liu S, Singla S, Dai Y, Mehta JL (2013) Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7 g. Int J Cardiol 168(2):1378–1385. doi:10.1016/j.ijcard.2012.12.045
Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386(6620):73–77. doi:10.1038/386073a0
Lee WJ, Ou HC, Hsu WC, Chou MM, Tseng JJ, Hsu SL, Tsai KL, Sheu WH (2010) Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg 52(5):1290–1300. doi:10.1016/j.jvs.2010.04.085
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H (2001) Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J 65(11):965–968
Hein S, Elsasser A, Kostin S, Zimmermann R, Schaper J (2002) Functional disturbances due to structural remodeling in the failing human heart. Arch Mal Coeur Vaiss 95(9):815–820
Ouimet M (2013) Autophagy in obesity and atherosclerosis: interrelationships between cholesterol homeostasis, lipoprotein metabolism and autophagy in macrophages and other systems. Biochim Biophys Acta 1831(6):1124–1133. doi:10.1016/j.bbalip.2013.03.007
Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB (2007) Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 14(1):146–157. doi:10.1038/sj.cdd.4401936
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2(5):270–278. doi:10.4161/oxim.2.5.9498
Park SJ, Kim YT, Jeon YJ (2012) Antioxidant dieckol downregulates the Rac1/ROS signaling pathway and inhibits Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma cells. Mol Cells 33(4):363–369. doi:10.1007/s10059-012-2285-2
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585(2–3):325–337. doi:10.1016/j.ejphar.2008.03.008
Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, Swarts M, Yang S, Zhang SB, Zhang K, Ju S, Olek DJ Jr, Schwartz L, Keng PC, Howell R, Zhang L, Okunieff P (2011) Antioxidant properties of quercetin. Adv Exp Med Biol 701:283–289. doi:10.1007/978-1-4419-7756-4_38
Boots AW, Wilms LC, Swennen EL, Kleinjans JC, Bast A, Haenen GR (2008) In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 24(7–8):703–710. doi:10.1016/j.nut.2008.03.023
Rogerio AP, Kanashiro A, Fontanari C, da Silva EV, Lucisano-Valim YM, Soares EG, Faccioli LH (2007) Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res 56(10):402–408. doi:10.1007/s00011-007-7005-6
Lee BH, Jeong SM, Lee JH, Kim JH, Yoon IS, Lee JH, Choi SH, Lee SM, Chang CG, Kim HC, Han Y, Paik HD, Kim Y, Nah SY (2005) Quercetin inhibits the 5-hydroxytryptamine type 3 receptor-mediated ion current by interacting with pre-transmembrane domain I. Mol Cells 20(1):69–73
Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, Kim HC, Park HY, Rhim H, Nah SY (2010) Quercetin enhances human alpha7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca(2+) binding sites. Mol Cells 30(3):245–253. doi:10.1007/s10059-010-0117-9
Chen L, Li J, Luo C, Liu H, Xu W, Chen G, Liew OW, Zhu W, Puah CM, Shen X, Jiang H (2006) Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features. Bioorganic Med Chem 14(24):8295–8306. doi:10.1016/j.bmc.2006.09.014
Davis JM, Murphy EA, McClellan JL, Carmichael MD, Gangemi JD (2008) Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol Regul Integr Comp Physiol 295(2):R505–R509. doi:10.1152/ajpregu.90319.2008
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC (2009) Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem 9(2):138–161
Puoci F, Morelli C, Cirillo G, Curcio M, Parisi OI, Maris P, Sisci D, Picci N (2012) Anticancer activity of a quercetin-based polymer towards HeLa cancer cells. Anticancer Res 32(7):2843–2847
Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW (2007) High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes 56(6):1559–1568. doi:10.2337/db06-1103
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24(2):288–293. doi:10.1161/01.ATV.0000114236.77009.06
Kim Y, Kim K, Lee H, Han S, Lee YS, Choe J, Kim YM, Hahn JH, Ro JY, Jeoung D (2009) Celastrol binds to ERK and inhibits FcepsilonRI signaling to exert an anti-allergic effect. Eur J Pharmacol 612(1–3):131–142. doi:10.1016/j.ejphar.2009.03.071
Nicholson SK, Tucker GA, Brameld JM (2008) Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc 67(1):42–47. doi:10.1017/S0029665108006009
Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G (2004) How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 80(1):15–21
Carracedo J, Merino A, Briceno C, Soriano S, Buendia P, Calleros L, Rodriguez M, Martin-Malo A, Aljama P, Ramirez R (2011) Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. FASEB J 25(4):1314–1322. doi:10.1096/fj.10-173377
Huang PH, Chen YH, Chen YL, Wu TC, Chen JW, Lin SJ (2007) Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome X. Heart 93(9):1064–1070. doi:10.1136/hrt.2006.107763
Acknowledgments
This work was supported by the Program of Science and Technology Commission of Shanghai Municipality 13ZR1414500 and 11ZR1433200; the Program of Putuo District Science and Technology Commission of Shanghai, No. B121.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Kangkang Zhi and Maoquan Li have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Autophagy inhibitors abrogated quercitrin-enhanced autophagy caused by ox-LDL. After incubation with 200 μg/ml ox-LDL for 2 h, cultured EPCs were treated 40 μM quercitrin for 6 h. (A) LC3-II fluorescent-stained cells and quantification of the number of autophagosomes. (B) Western blot analysis of LC3-I/II and Beclin-1 expression. β-actin was included as a loading control (n=3, *p < 0.05, compared with ox-LDL group; # p < 0.05, compared with 40 μM quercitrin group). (TIFF 753 kb)
Rights and permissions
About this article
Cite this article
Zhi, K., Li, M., Bai, J. et al. Quercitrin treatment protects endothelial progenitor cells from oxidative damage via inducing autophagy through extracellular signal-regulated kinase. Angiogenesis 19, 311–324 (2016). https://doi.org/10.1007/s10456-016-9504-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-016-9504-y