Abstract
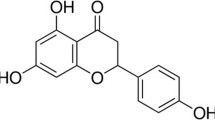
The flavonoid quercetin is a low molecular weight substance found in fruits and vegetables. Aside from its anti-oxidative effect, quercetin, like other flavonoids, has a wide range of neuropharmacological actions. The α7 nicotinic acetylcholine receptor (α7 nAChR) has a Ca2+-binding site, is highly permeable to the Ca2+ ion, and plays important roles in Ca2+-related normal brain functions. Dysfunctions of α7 nAChR are associated with a variety of neurological disorders. In the present study, we investigated the effects of quercetin on the ACh-induced inward peak current (I ACh ) in Xenopus oocytes that heterologously express human α7 nAChR. I ACh was measured with the two-electrode voltage clamp technique. In oocytes injected with α7 nAChR cRNA, the effects of the co-application of quercetin on I ACh were concentration-dependent and reversible. The ED50 was 36.1 + 6.1 μM. Quercetin-mediated enhancement of I ACh caused more potentiation when quercetin was pre-applied. The degree of I ACh potentiation by quercetin pre-application was time-dependent and saturated after 1 min. Quercetin-mediated I ACh enhancement was not affected by ACh concentration and was voltage-independent. However, quercetin-mediated I ACh enhancement was dependent on extracellular Ca2+ concentrations and was specific to the Ca2+ ion, since the removal of extracellular Ca2+ or the addition of Ba2+ instead of Ca2+ greatly diminished quercetin enhancement of IACh. The mutation of Glu195 to Gln195, in the Ca2+-binding site, almost completely diminished quercetin-mediated I ACh enhancement. These results indicate that quercetin-mediated I ACh enhancement human α7 nAChR heterologously expressed in Xenopus oocytes could be achieved through interactions with the Ca2+-binding site of the receptor.
Similar content being viewed by others
References
Amador, M., and Dani, J.A. (1995). Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. J. Neurosci. 15, 4525–4532.
Berg, D.K., and Conroy, W.G. (2002). Nicotinic alpha7 receptors: synaptic options and downstream signaling in neurons. J. Neurobiol. 53, 512–523.
Bertrand, D., Galzi, J.L., Devillers-Thiéry, A., Bertrand, S., and Changeux, J.P. (1993).Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. USA 90, 6971–6975.
Boulter, J., Evans, K., Goldman, D., Martin, G., Treco, D., Heinemann, S., and Patrick, J. (1986). Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alphasubunit. Nature 319, 368–374.
Boulter, J., Connolly, J., Deneris, E., Goldman, D., Heinemann, S., and Patrick, J. (1987). Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl. Acad. Sci. USA 84, 7763–7767.
Brumwell, C.L., Johnson, J.L., and Jacob, M.H. (2002). Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J. Neurosci. 22, 8101–8109.
Castro, N.G., and Albuquerque, E.X. (1995). alpha-Bungarotoxinsensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys. J. 68, 516–524.
Changeux, J.P., and Edelstein, S.J. (2001). Allosteric mechanisms in normal and pathological nicotinic acetylcholine receptors. Curr. Opin. Neurobiol. 11, 369–377.
Charpantier, E., Wiesner, A., Huh, K.H., Ogier, R., Hoda, J.C., Allaman, G., Raggenbass, M., Feuerbach, D., Bertrand, D., and Fuhrer, C. (2005). Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J. Neurosci. 25, 9836–9849.
Chavez-Noriega, L.E., Crona, J.H., Washburn, M.S., Urrutia, A., Elliott, K.J., and Johnson, E.C. (1997). Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 280, 346–356.
Chini, B., Raimond, E., Elgoyhen, A.B., Moralli, D., Balzaretti, M., and Heinemann, M. (1994). Molecular cloning and chromosomal localization of the human alpha 7-nicotinic receptor subunit gene (CHRNA7). Genomics 19, 379–381.
Cho, C.H., Song, W., Leitzell, K., Teo, E., Meleth, A.D., Quick, M.W., and Lester, R.A. (2005). Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J. Neurosci. 25, 3712–3723.
Couturier, S., Bertrand, D., Matter, J.M., Hernandez, M.C., Bertrand, S., Millar, N., Valera, S., Barkas, T., and Ballivet, M. (1990). A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homooligomeric channel blocked by α-BTX. Neuron 5, 847–856.
Dajas-Bailador, F., and Wonnacott, S. (2004). Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 25, 317–324.
Dajas-Bailador, F.A., Soliakov, L., and Wonnacott, S. (2002). Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 80, 520–530.
Dascal, N. (1987). The use of Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 22, 317–387.
Eddins, D., Lyford, L.K., Lee, J.W., Desai, S.A., and Rosenberg, R.L. (2002). Permeant but not impermeant divalent cations enhance activation of nondesensitizing alpha(7) nicotinic receptors. Am. J. Physiol. Cell. Physiol. 282, 796–804.
Eiselé, J.L., Bertrand, S., Galzi, J.L., Devillers-Thiéry, A., Changeux, J.P., and Bertrand, D. (1993). Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 366, 479–483.
Elgoyhen, A.B., Johnson, D.S., Boulter, J., Vetter, D.E., and Heinemann, S. (1994). α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79, 705–715
Galzi, J.L., Devillers-Thiéry, A., Hussy, N., Bertrand, S., Changeux, J.P., and Bertrand, D. (1992). Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature 359, 500–505.
Gilbert, D., Lecchi, M., Arnaudeau, S., Bertrand, D., and Demaurex, N. (2009). Local and global calcium signals associated with the opening of neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium 45, 198–207.
Gotti, C., and Clementi, F. (2004). Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 74, 363–396.
Gotti, C., Hanke, W., Maury, K., Moretti, M., Ballivet, M., Clementi, F., and Bertrand, D. (1994). Pharmacology and biophysical properties of α7 and α7–α8 α-bungarotoxin receptor subtypes immunopurified from the chick optic lobe. Eur. J. Neurosci. 6, 1281–1291.
Gotti, C., Carbonnelle, E., Moretti, M., Zwart, R., and Clementi, F. (2000). Drugs selective for nicotinic receptor subtypes: a real possibility or a dream? Behav. Brain Res. 113, 183–192.
Jensen, M.L., Schousboe, A., and Ahring, P.K. (2005). Charge selectivity of the Cys-loop family of ligand-gated ion channels. J. Neurochem. 92, 217–225.
Karlin, A. (2002). Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114.
Khiroug, L., Giniatullin, R., Klein, R.C., Fayuk, D., and Yakel, J.L. (2003). Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J. Neurosci. 23, 9024–9031.
Kihara, T., Shimohama, S., Sawada, H., Honda, K., Nakamizo, T., Shibasaki, H., Kume, T., and Akaike, A. (2001). Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J. Biol. Chem. 276, 13541–13546.
Klein, R.C., and Yakel, J.L. (2005). Paired-pulse potentiation of alpha7-containing nAChRs in rat hippocampal CA1 stratum radiatum interneurones. J. Physiol. 568, 881–889.
Lee, B.H., Jeong, S.M., Lee, J.H., Kim, J.H., Yoon, I.S., Lee, J.H., Choi, S.H., Lee, S.M., Chang, C.G., Kim, H.C., et al. (2005). Quercetin inhibits the 5-hydroxytryptamine type 3 receptormediated ion current by interacting with pre-transmembrane domain I. Mol. Cells 20, 69–73.
Lee, B.H., Lee, J.H., Yoon, I.S., Lee, J.H., Choi, S.H., Pyo, M.K., Jeong, S.M., Choi, W.S., Shin, T.J., Lee, S.M., et al. (2007). Human glycine alpha1 receptor inhibition by quercetin is abolished or inversed by alpha267 mutations in transmembrane domain 2. Brain Res. 1161, 1–10.
Lee, B.H., Choi, S.H., Pyo, M.K., Shin, T.J., Hwang, S.H., Kim, B.R., Lee, S.M., Lee, J. H., Lee, J.H., Lee, H.S., et al. (2009). A role for Leu247 residue within transmembrane domain 2 in ginsenoside-mediated alpha7 nictoninc acetylcholine receptor regulation. Mol. Cells 27, 591–599.
Lena, C., and Changeux, J.P. (1997). Pathological mutations of nicotinic receptors and nicotine-based therapies for brain disorders. Curr. Opin. Neurobiol. 7, 674–682.
Le Novère, N., Grutter, T., and Changeux, J.P. (2002). Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc. Natl. Acad. Sci. USA 99, 3210–3215.
Nashmi, R., and Lester, H.A. (2006). CNS localization of neuronal nicotinic receptors. J. Mol. Neurosci. 30, 181–184.
Palma, E., Mileo, A.M., Eusebi, F., and Miledi, R. (1996). Threonine-for-leucine mutation within domain M2 of the neuronal alpha7 nicotinic receptor convert 5-hydroxytryptamine from antagonist to agonist. Proc. Natl. Acad. Sci. USA 93, 11231–11235.
Pullan, L.M., Olney, J.W., Price, M.T., Compton, R.P., Hood, W.F., Michel, J., and Monahan, J.B. (1987). Excitatory amino acid receptor potency and subclass specificity of sulfur-containing amino acids. J. Neurochem. 49, 1301–1307.
Revah, F., Bertrand, D., Galzi, J.L., Devillers-Thiéry, A., Mulle, C., Hussy, N., Bertrand, S., Ballivet, M., and Changeux, J.P. (1991). Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849.
Séguéla, P., Wadiche, J., Dineley-Miller, K., Dani, J.A., and Patrick, J.W. (1993). Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 13, 596–604.
Sine, S.M., Claudio, T., and Sigworth, F.J. (1990). Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J. Gen. Physiol. 96, 395–437.
Vernino, S., Amador, M., Luetje, C.W., Patrick, J., and Dani, J.A. (1992). Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8, 127–134.
Weiland, S., Bertrand, D., and Leonard, S. (2000). Neuronal nicotinic acetylcholine receptors: from the gene to the disease. Behav. Brain Res. 113, 43–56.
Yao, Y., Han, D.D., Zhang, T., and Yang, Z. (2010). Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytother. Res. 24, 136–140.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
About this article
Cite this article
Lee, BH., Choi, SH., Shin, TJ. et al. Quercetin enhances human α7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca2+ binding sites. Mol Cells 30, 245–253 (2010). https://doi.org/10.1007/s10059-010-0117-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-010-0117-9