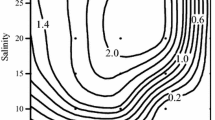
Abstract
The Carpathian Basin hosts a number of small, shallow, saline, alkaline ponds. As being endorheic basins, they are highly threatened by the climate change and response of biota to changing climate has been largely unexplored. We investigated the effects of salinity changes on the photosynthetic activity of Nitzschia frustulum, which is one of the main dominant taxa of the saline lakes in the Fertő-Hanság Region of the Carpathian Basin. The photosynthetic activity of the species was measured along temperature (5, 10, 15, 20, 25, 30, 35, 40 °C), light (0–8–35–70–110–200–400–800–1200 µmol m−2 s−1), SO4 2− (0–50–600–1200–2400–3600–4800 mg L−1) and Cl− (0–36–437.5–875–1750–3500–5250 mg L−1) gradients under laboratory conditions in photosynthetron. The conductivity optimal of N. frustulum was around 5600 µS cm−1 with wide salinity tolerance. The species preferred the HCO3 −–SO4 2−-type waters since its photosynthetic activity (3.62 mg C mg Chl-a −1 h−1) was more than twice higher than in HCO3 −–Cl−-type media. Its photosynthesis saturated at very low-light intensity, and photoinhibition was not observed during the experiments. The maximal photosynthesis was measured at 28–29 °C. However, above 30 °C, the decline of photosynthesis of N. frustulum can be forecasted.









Similar content being viewed by others
References
Ahlgren G (1987) Temperature functions in biology and their application to algal growth constants. Oikos 49:177–190. doi:10.2307/3566025
Anneville O, Domaizon I, Kerimoglu O, Rimet F, Jacquet S (2015) Blue-green algae in a “Greenhouse Century”? New insights from field data on climate change impacts on cyanobacteria abundance. Ecosystems 18:441–458
APHA (1998) Standard methods for the examination of water and wastewater. United Book Press, Inc., Baltimore
Barker HA (1935) Photosynthesis in diatoms. Archiv für Mikrobiologie 6:141–156. doi:10.1007/bf00407284
Bauld J (1981) Occurrence of benthic microbial mats in saline lakes. In: Williams WD (ed) Salt Lakes, vol 5. Developments in hydrobiology. Springer, pp 87–111. doi:10.1007/978-94-009-8665-7_8
Belay A, Fogg GE (1978) Photoinhibition of photosynthesis in Asterionella formosa (Bacillariophyceae). J Phycol 14:341–347. doi:10.1111/j.1529-8817.1978.tb00310.x
Blinn DW (1993) Diatom community structure along physicochemical gradients in saline lakes. Ecology 74:1246–1263. doi:10.2307/1940494
Boros E, Molnár A, Olajos P, Takács A, Jakab G, Dévai G (2006) Nyílt vízfelszínű szikes élőhelyek elterjedése, térinformatikai adatbázisa és természetvédelmi helyzete a Pannon biogeográfiai régióban. Geographical distribution, GIS database and nature conservation status of opened sodic (alkaline) water bodies in Pannonic Biogeographical Region). Hidrológiai Közlöny 86:146–147
Boros E, Horváth Z, Wolfram G, Vörös L (2014) Salinity and ionic composition of the shallow astatic soda pans in the Carpathian Basin. Ann Limnol Int J Limnol 50:59–69
Boyer J (1976) Water deficits and photosynthesis. In: Kozlowsky TT (ed) Water deficits and plant growth, vol 4. Academic Press, Inc., London, pp 153–190
Brotas V, Catarino F (1995) Microphytobenthos primary production of Tagus estuary intertidal flats (Portugal). Neth J Aquatic Ecol 29:333–339. doi:10.1007/bf02084232
Busse S, Jahn R, Schulz C-J (1999) Desalinization of running waters: II. Benthic diatom communities: A comparative field study on responses to decreasing salinities. Limnol Ecol Manag Inland Waters 29:465–474. doi:10.1016/S0075-9511(99)80053-X
Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425:365
Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW (2010) Soil microbial community responses to multiple experimental climate change drivers. Appl Environ Microbiol 76:999–1007. doi:10.1128/aem.02874-09
Christensen J, Christensen O (2007) A summary of the PRUDENCE model projections of changes in European climate by the end of this century. Clim Change 81:7–30. doi:10.1007/s10584-006-9210-7
Cole J, Howarth R, Nolan S, Marino R (1986) Sulfate inhibition of molybdate assimilation by planktonic algae and bacteria: some implications for the aquatic nitrogen cycle. Biogeochemistry 2:179–196. doi:10.1007/bf02180194
Cumming B, Smol J (1993) Development of diatom-based salinity models for paleoclimatic research from lakes in British Columbia (Canada). In: van Dam H (ed) Twelfth international diatom symposium, vol 90. Developments in hydrobiology. Springer, pp 179–196. doi:10.1007/978-94-017-3622-0_20
Dauta A, Devaux J, Piquemal F, Boumnich L (1990) Growth rate of four freshwater algae in relation to light and temperature. Hydrobiologia 207:221–226. doi:10.1007/bf00041459
De Deckker P (1988) Biological and sedimentary facies of Australian salt lakes. Palaeogeogr Palaeoclimatol Palaeoecol 62:237–270. doi:10.1016/0031-0182(88)90056-9
de Tezanos Pinto P, Litchman E (2010) Eco-physiological responses of nitrogen-fixing cyanobacteria to light. Hydrobiologia 639:63–68. doi:10.1007/s10750-009-0014-4
Dokulil M (2013) Impact of climate warming on European inland waters. Inland Waters 4:27–40
El-Sabaawi R, Harrison PJ (2006) Interactive effects of irradiance and temperature on the photosynthetic physiology of the pennate diatom Pseudo-nitzschia granii (Bacillariophyceae) from the northeast subarctic Pacific. J Phycol 42:778–785. doi:10.1111/j.1529-8817.2006.00246.x
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Princeton University Press, Oxford
Fiala M, Oriol L (1990) Light-temperature interactions on the growth of Antarctic diatoms. Polar Biol 10:629–636. doi:10.1007/bf00239374
Fritz SC, Juggins S, Battarbee RW (1993) Diatom assemblages and ionic characterization of lakes of the Northern Great Plains, North America: a tool for reconstructing past salinity and climate fluctuations. Can J Fish Aquat Sci 50:1844–1856. doi:10.1139/f93-207
Gasse F, Juggins S, Khelifa LB (1995) Diatom-based transfer functions for inferring past hydrochemical characteristics of African lakes. Palaeogeogr Palaeoclimatol Palaeoecol 117:31–54. doi:10.1016/0031-0182(94)00122-O
George G, Hurley M, Hewitt D (2007) The impact of climate change on the physical characteristics of the larger lakes in the English Lake District. Freshw Biol 52:1647–1666. doi:10.1111/j.1365-2427.2007.01773.x
Goudie AS (2003) Great warm deserts of the world: landscapes and evolution. Oxford University Press, Oxford
Grant W (2006) Alkaline environments and biodiversity. In: Gerday C, Glansdorff N (eds) Extremophiles. Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO. UNESCO, Eolss, Oxford
Gyllström M et al (2005) The role of climate in shaping zooplankton communities of shallow lakes. Limnol Oceanogr 50:2008–2021
Hammer UT (1981) Primary production in saline lakes. In: Williams WD (ed) Salt Lakes, vol 5. Developments in hydrobiology. Springer, pp 47–57. doi:10.1007/978-94-009-8665-7_5
Hammer UT (1986) Saline lake ecosystems of the world, vol 59. Springer, Dordrecht
Hammer UT, Shamess J, Haynes R (1983) The distribution and abundance of algae in saline lakes of Saskatchewan, Canada. Hydrobiologia 105:1–26. doi:10.1007/bf00025173
Harley CDG et al (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241. doi:10.1111/j.1461-0248.2005.00871.x
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Physiol Plant Mol Biol 51:463–499. doi:10.1146/annurev.arplant.51.1.463
Hill W (1996) Effects of light. In: Stevenson R, Bothwell ML, Lowe RL (eds) Algal ecology, freshwater benthic ecosystem. Academic Press, San Diego, pp 121–148
Hopkins W, Hüner N (2004a) Plant Environmental Stress Physiology. In: Hopkins W, Hüner N (eds) Introduction to Plant Physiology, 3rd edn. Wiley, Danvers, pp 459–493
Hopkins W, Hüner N (2004b) Plants and Inorganic Nutrients, 3rd edn. Wiley, Danvers
Horváth Z, Vad CF, Vörös L, Boros E (2013) The keystone role of anostracans and copepods in European soda pans during the spring migration of waterbirds. Freshw Biol 58:430–440. doi:10.1111/fwb.12071
Ionescu V, Năstăsescu M, Spiridon L, Bulgăreanu VC (1998) The biota of Romanian saline lakes on rock salt bodies: a review. Int J Salt Lake Res 7:45–80. doi:10.1023/a:1009025228069
Jarman AOH, Jones ED (1982) Llyfr Du Caerfyrddin. Gwasg Prifysgol Cymru, Cardiff
Kemp AES, Pike J, Pearce RB, Lange CB (2000) The “Fall dump”—a new perspective on the role of a “shade flora” in the annual cycle of diatom production and export flux. Deep Sea Res Part II Top Stud Oceanogr 47:2129–2154. doi:10.1016/S0967-0645(00)00019-9
Keresztes ZG et al (2012) First record of picophytoplankton diversity in Central European hypersaline lakes. Extremophiles 16:759–769. doi:10.1007/s00792-012-0472-x
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge
Kirst G (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Biol 41:21–53
Krammer K, Lange-Bertalot H (1997) Süßwasserflora von Mitteleuropa. vol Band 2/2: Bacillariaceae, Epithemiaceae, Surirellaceae. Bacillariophyceae. Gustav Fischer Verlag, Heidelberg
Krumbein WE, Cohen Y, Shilo M (1977) Solar lake (Sinai). 4. Stromatolitic cyanobacterial mats. Limnol Oceanogr 22:635–655
Leatherbarrow R (2009) GraFit Data Analysis Software for Windows, 7.0.3 edn. Erithacus Software Ltd., Horley
Lehman JT, Botkin DB, Likens GE (1975) The assumptions and rationales of a computer model of phytoplankton population dynamics. Limnol Oceanogr 20:343–364
Litchman E, Klausmeier CA (2008) Trait-based community ecology of phytoplankton. Annu Rev Ecol Evol Syst 39:615–639
Mann DG (1999) The species concept in diatoms. Phycologia 38:437–495. doi:10.2216/i0031-8884-38-6-437.1
Mason IM, Guzkowska MAJ, Rapley CG, Street-Perrott FA (1994) The response of lake levels and areas to climatic change. Clim Change 27:161–197. doi:10.1007/bf01093590
Molinero JC, Anneville O, Souissi S, Lainé L, Gerdeaux D (2007) Decadal changes in water temperature and ecological time-series in Lake Geneva, Europe-detecting relationships with the subtropical Atlantic climate variability. Clim Res 34:15–23
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. doi:10.1046/j.0016-8025.2001.00808.x
Nelson DM, Tréguer P, Brzezinski MA, Leynaert A, Quéguiner B (1995) Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob Biogeochem Cycles 9:359–372. doi:10.1029/95gb01070
Oppenheim DR (1991) Seasonal changes in epipelic diatoms along an intertidal shore, Berrow Flats, Somerset. J Mar Biol Assoc UK 71:579–596. doi:10.1017/S0025315400053169
Padisák J (2004) Phytoplankton. In: O’Sullivan P, Reynolds CS (eds) The lakes handbook, vol 1., Limnology and limnotic ecologyBlackwell, Oxford, pp 251–309
Pálffy K et al (2014) Unique picoeukaryotic algal community under multiple environmental stress conditions in a shallow, alkaline pan. Extremophiles 18:111–119
Pan Y, Rao DVS, Mann KH (1996) Acclimation to low light intensity in photosynthesis and growth of Pseudo-nitzschia multiseris Hasle, a neurotoxigenic diatom. J Plankton Res 18:1427–1438. doi:10.1093/plankt/18.8.1427
Passy SI (2007) Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat Bot 86:171–178. doi:10.1016/j.aquabot.2006.09.018
Pinckney JL, Zingmark R (1991) Effects of tidal stage and sun angles on intertidal benthic microalgal productivity. Mar Ecol Prog Ser 76:81
Platt T, Gallegos C, Harrison W (1981) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:103–111
Radchenko I, Il’Yash L (2006) Growth and photosynthetic activity of diatom Thalassiosira weissflogii at decreasing salinity. Biol Bull 33:242–247
Reynolds C (1988) Functional morphology and the adaptive strategies of freshwater phytoplankton. Growth and reproductive strategies of freshwater phytoplankton. Cambridge University Press, Cambridge, pp 388–433
Reynolds C, Kinne O (1997) Excellence in ecology. In: Kinne O (ed) Vegetation process processes in the pelagic model for ecosystem theory. Ecology Institute, Oldendorf/Luhe
Roessig J, Woodley C, Cech J Jr, Hansen L (2004) Effects of global climate change on marine and estuarine fishes and fisheries. Rev Fish Biol Fish 14:251–275. doi:10.1007/s11160-004-6749-0
Roubeix V, Lancelot C (2008) Effect of salinity on growth, cell size and silicification of an euryhaline freshwater diatom: Cyclotella meneghiniana Kütz. Trans Waters Bull 2:31–38
Roux M, Servant-Vildary S, Servant M (1991) Inferred ionic composition and salinity of a Bolivian Quaternary lake, as estimated from fossil diatoms in the sediments. Hydrobiologia 210:3–18. doi:10.1007/bf00014319
Saros JE, Fritz SC (2002) Resource competition among saline-lake diatoms under varying N/P ratio, salinity and anion composition. Freshw Biol 47:87–95. doi:10.1046/j.1365-2427.2002.00781.x
Sarthou G, Timmermans KR, Blain S, Tréguer P (2005) Growth physiology and fate of diatoms in the ocean: a review. J Sea Res 53:25–42. doi:10.1016/j.seares.2004.01.007
Schlösser UG (1994) SAG—Sammlung von Algenkulturen at the University of Göttingen Catalogue of Strains 1994. Bot Acta 107:113–186. doi:10.1111/j.1438-8677.1994.tb00784.x
Servant Vildary S (1984) Les diatomées des lacs sursalés boliviens: sous-classe Pennatophycidées: 1. Famille des Nitzschiacées. Cahiers ORSTOM Série Géologie 14:35–53
Servant-Vildary S, Roux M (1990) Multivariate analysis of diatoms and water chemistry in Bolivian saline lakes. In: Comín F, Northcote T (eds) Saline Lakes, vol 59. Developments in hydrobiology. Springer, pp 267–290. doi:10.1007/978-94-009-0603-7_23
Smol JP, Walker IR, Leavitt PR (1991) Paleolimnology and hindcasting climatic trends. Verhandlungen der Internationale Vereinigung für theoretische und angewandte Limnologie 24:1240–1246
Sobrino C, Neale PJ (2007) Short-term and long-term effects of temperature on photosynthesis in the diatom Thalassiosira pseudonana under UVR exposure. J Phycol 43:426–436
Somogyi B, Vörös L, Pálffy K, Székely G, Bartha C, Keresztes Z (2014) Picophytoplankton predominance in hypersaline lakes (Transylvanian Basin, Romania). Extremophiles 18:1075–1084. doi:10.1007/s00792-014-0685-2
Stenger-Kovács C et al (2014) Vanishing world: alkaline, saline lakes in Central Europe and their diatom assemblages. Inland Waters 4(383):396
Stramski D, Sciandra A, Claustre H (2002) Effects of temperature, nitrogen, and light limitation on the optical properties of the marine diatom Thalassiosira pseudonana. Limnol Oceanogr 47:392–403
Sudhir P, Murthy SDS (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486. doi:10.1007/s11099-005-0001-6
Sullivan MJ, Currin CA (2000) Community structure and functional dynamics of benthic microalgae in salt marshes. In: Weinstein M, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Springer, New York, pp 81–106
Taylor WR (1964) Light and photosynthesis in intertidal benthic diatoms. Helgoländer Meeresun 10:29–37. doi:10.1007/bf01626096
Team RDC (2010) R: A language and environment for statistical computing, 2.11.0 edn. R Foundation for Statistical Computing, Vienna
Trobajo R, Cox EJ, Quintana XD (2004) The effects of some environmental variables on the morphology of Nitzschia frustulum (Bacillariophyta), in relation its use as a bioindicator. Nova Hedwigia 79:433–445. doi:10.1127/0029-5035/2004/0079-0433
Trobajo R, Rovira L, Ector L, Wetzel CE, Kelly M, Mann DG (2012) Morphology and identity of some ecologically important small Nitzschia species. Diatom Res 28:37–59. doi:10.1080/0269249x.2012.734531
Underwood GJC (1994) Seasonal and spatial variation in epipelic diatom assemblages in the severn estuary. c Res 9:451–472. doi:10.1080/0269249x.1994.9705319
Underwood G, Phillips J, Saunders K (1998) Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur J Phycol 33:173–183. doi:10.1080/09670269810001736673
Üveges V, Vörös L, Padisák J, Kovács A (2011) Primary production of epipsammic algal communities in Lake Balaton (Hungary). Hydrobiologia 660:17–27. doi:10.1007/s10750-010-0396-3
V.-Balogh K, Németh B, Vörös L (2009) Specific attenuation coefficients of optically active substances and their contribution to the underwater ultraviolet and visible light climate in shallow lakes and ponds. Hydrobiologia 632:91–105. doi:10.1007/s10750-009-9830-9
Veres AJ, Pienitz R, Smol JP (1995) Lake water salinity and periphytic diatom succession in three subarctic lakes, Yukon Territory, Canada. Arctic 48:63–70
Vörös L, Boros E (2010) Nodularia willei Gardn. tömegprodukció: a planktonikus és bentonikus elsődleges termelés peremfeltételei egy kiskunsági szikes tóban (Kelemen-szék). Acta Biologica Debrecina Oecologia Hungarica 22:139–152
Warren JK (2006) Depositional chemistry and hydrology. In: Evaporites: sediments, resources and hydrocarbons. pp 59–138
Wetzel RG, Likens GE (2000) Limnological analyses. Springer, New York
Whitney DE, Darley WM (1983) Effect of light intensity upon salt marsh benthic microalgal photosynthesis. Mar Biol 75:249–252. doi:10.1007/bf00406009
Wilhelm S, Hintze T, Livingstone DM, Adrian R (2006) Long-term response of daily epilimnetic temperature extrema to climate forcing. Can J Fish Aquat Sci 63:2467–2477. doi:10.1139/f06-140
Williams WD (1981) Inland salt lakes: an introduction. Hydrobiologia 81–82:1–14. doi:10.1007/bf00048701
Williams W (2005) Lakes in arid environments. In: O’Sullivan P, Reynolds C (eds) The lakes handbook, vol 2., Lake restoration and rehabilitationBlackwell, Oxford, pp 200–240
Wilson S, Cumming B, Smol J (1994) Diatom-salinity relationships in 111 lakes from the Interior Plateau of British Columbia, Canada: the development of diatom-based models for paleosalinity reconstructions. J Paleolimnol 12:197–221. doi:10.1007/bf00678021
Ziemann H (1971) Die Wirkung des Salzgehaltes auf die Diatomeenflora als Grundlage für eine biologische Analyse und Klassifikation der Binnengewässer. Limnologica 8:505–525
Ziemann H (1982) Indikatoren für den Salzgehalt der Binnengewässer- Halobiensystem. In: Bremg G, Tümpling WV (eds) Ausgewählte Methoden der Gewässeruntersuchung, vol II. Biologische, mikrobiologische und toxikologische Methoden. Jena
Ziemann H, Kies L, Schulz C-J (2001) Desalinization of running waters: III. Changes in the structure of diatom assemblages caused by a decreasing salt load and changing ion spectra in the river Wipper (Thuringia, Germany). Limnol Ecol Manag Inland Waters 31:257–280. doi:10.1016/S0075-9511(01)80029-3
Acknowledgments
We thank Dr. Boglárka Somogyi and Éva Koltai for their advice in culture methods, Dávid Németh for his technical assistance, Dr. Krisztina Buczkó for the electron microscopic photographs, the colleagues of the Fertő-Hanság Hungarian National Park Attila Pellinger, András Ambrus and Péter Kugler for their assistance in sampling. The study was supported by the Hungarian National Science Foundation (OTKA K81599).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Rights and permissions
About this article
Cite this article
Lengyel, E., Kovács, A.W., Padisák, J. et al. Photosynthetic characteristics of the benthic diatom species Nitzschia frustulum (Kützing) Grunow isolated from a soda pan along temperature-, sulfate- and chloride gradients. Aquat Ecol 49, 401–416 (2015). https://doi.org/10.1007/s10452-015-9533-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-015-9533-4