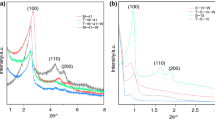
Abstract
Mesoporous adsorbent, SMCM-41, was synthesized from a new amorphous silica (MPI) obtained from sand, at low cost, with the aim of CO2 adsorption. To compare the properties of the SMCM-41, was used a mesoporous MCM-41 synthesized from commercial silica. Other adsorbents were obtained by impregnation of the materials MCM-41 and SMCM-41 using mono and diethanolamine amines, MEA (M) and DEA (D) at different concentrations: 3 % M, 3 % D, 10 % M, and 3 % M + 3 % D, to study the effect of concentration and the chain length of the amines in the CO2 adsorption performance. The synthesized materials were characterized by X-ray diffraction, N2 adsorption/desorption, thermogravimetric analysis (TG/DTG), scanning electron microscopy and transmission electron miscroscopy, to confirm the structural properties. CO2 adsorption isotherms were obtained at 25 °C and pressures from 1 to 40 bar. The experimental results were fitted to theoretical models of Langmuir and Freundlich, showing favorable CO2 adsorption for all materials. The maximum adsorption amount was 11.39 mmol g−1 for MCM-41 and 10.40 mmol g−1 for SMCM-41. It was found that for pressures close to 1 bar, impregnation with 3 % MEA was most effective with twice the CO2 adsorption compared to the pure material MCM-41 and SMCM-41. The impregnation with DEA was not efficient for the adsorption of CO2, confirming the negative effect of the size of the organic chain. This paper presents the mesoporous material SMCM-41, which has low cost, with high potential for adsorption of CO2 in different conditions of pressure.








Similar content being viewed by others
References
Aiello, D., Mirabelli, I., Testa, F.: Adsorption of 2-methylbenzoic acid onto MCM-41 mesoporous material: kinetics and equilibrium studies. J. Solid Gel. Sci. Technol. 64, 1–8 (2012)
Allen, S.J., Mckay, G., Porter, J.F.: Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 280, 322–333 (2004)
Almeida, R.M., Guiton, T.A., Pantano, C.G.: Characterization of silica gels by infrared reflection spectroscopy. J. Non Cryst. Solids 121, 193–197 (1990)
Beck, J.S., Vartulli, J.C., Roth, W.J., Leonowicz, M.E., Kresge, C.T., Schmitt, K.D., Chu, C.T.-W., Olson, D.H., Sheppard, E.W., McCullen, S.B., Higgins, J.B., Schlenkert, J.L.: A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 114, 10834–10843 (1992)
Belmabkhout, Y., Serna-Guerrero, R., Sayari, A.: Adsorption of CO2-containing gás mixtures over amine-bearing pore-expanded MCM-41 silica: application for CO2 separation. Adsorption 17, 395–401 (2011)
Bhagiyalakshmi, M., Yun, L.J., Anuradha, R., Jang, H.T.: Utilization of rice husk ash as silica source for the synthesis of mesoporous sílicas and their application to CO2 adsorption through TREN/TEPA grafting. J. Hazard. Mater. 175, 928–938 (2010)
Bounaceur, R., Lape, N., Roizard, D., Vallieres, C., Favre, E.: Membrane processes for post-combustion carbon dioxide capture: a parametric study. Energy 31, 2556–2570 (2006)
Braga, R.M., Barros, J.M.F., Melo, D.M.A., Melo, M.A.F., Aquino, F.M., Freitas, J.C.O., Santiago, R.C.: Kinetic study of template removal of MCM-41 derived from rice husk ash. J. Therm. Anal. Calorim. 111, 1013–1018 (2013)
Cauvel, A., Renard, G., Brunel, D.: Monoglyceride synthesis by heterogeneous catalysis using MCM-41 type silicas functionalized with amino groups. J. Org. Chem. 62, 749–751 (1997)
Chalal, N., Bouhali, H., Hamaizi, H., Lebeau, B., Bengueddach, A.: CO2 sorption onto silica mesoporous materials made from nonionic surfactants. Microporous Mesoporous Mater. 210, 32–38 (2015)
Chang, F.-Y., Chao, K.-J., Cheng, H.-H., Tan, C.-S.: Adsorption of CO2 on to amine-grafted mesoporous sílicas. Sep. Purif. Technol. 70, 87–95 (2009)
Chen, C., Son, W.-J., You, K.-S., Ahn, J.-W., Ahn, W.-S.: Carbon dioxide capture using amine-impregnated HMS having textural mesoporosity. Chem. Eng. J. 161, 46–52 (2010)
European Commission—A vision for zero emission fossil fuel power plants. Luxembourg: office for official publications of the European communities EUR 22043, http://ec.europa.eu/research/energy/pdf/zero_emission_ffpp_en.pdf (2006). Accessed Jun 2015
Finsy, V., Ma, L., Alaerts, L., DeVos, D.E., Baron, G.V., Denayer, J.F.M.: Separation of CO2/CH4 mixtures with the MIL-53(Al) metal-organic framework. Microporous Mesoporous Mater. 120, 221–227 (2009)
Freundlich, H.: Uber die adsorption in Losungen. Zeitschrift fur Physikalis-¨che. CEIME 57, 385–470 (1907)
Fujiki, J., Yamada, H., Yogo, K.: Enhanced adsorption of carbon dioxide on surface-modified mesoporous silica-supported tetraethylenepentamine: role of surface chemical structure. Microporous Mesoporous Mater. 215, 76–83 (2015)
Goscianska, J., Olejnik, A., Pietrzak, R.: Comparison of ordered mesoporous materials sorption properties towards amino acids. Adsorption 19, 581–588 (2013)
Gouedarda, C., Picqa, D., Launay, F., Carrette, P.L.: Amine degradation in CO2 capture. I. A review. Int J Greenhouse Gas Control. 10, 224–270 (2012)
Goworek, J., Borówka, A., Zaleski, R., Kusak, R.: Template transformations in preparation of MCM-41 silica. J. Therm. Anal. Calorim. 79, 555–560 (2005)
Guan-Sajonz, H., Guiochon, G., Davis, E., Gulakowski, K., Smith, D.W.: Study of the physico-chemical properties of some packing materials III. Pore size and surface area distribution. J. Chromatogr. A 773, 33–51 (1997)
International Energy Agency (IEA), World Energy Outlook 2006. OECD/IEA, 2006. Report, Paris, France. https://www.iea.org/publications/freepublications/publication/weo2006.pdf (2006). Accessed on Jun 2015
Kalapathy, U., Proctor, A., Shultz, J.: An improved method for production of silica from rice hull ash. Bioresour. Technol. 85, 285–289 (2002)
Kamarudin, K.S.N., Alias, N.: Adsorption performance of MCM-41 impregnated with amine for CO2 removal. Fuel Process. Technol. 106, 332–337 (2013)
Knowles, G.P., Delaney, S.W., Chaffee, A.L.: Diethylenetriamine [propyl(silyl)]—functionalized (DT) mesoporous silicas as CO2 adsorbents. Ind. Eng. Chem. Res. 45(8), 2626–2633 (2006)
Koh, C.A., Nooney, R., Tahir, S.: Characterization and catalytic properties of MCM-41 and Pd/MCM-41 materials. Catal. Lett. 47, 199–203 (1997)
Kresge, C.T., Leonowicz, M.E., Roth, W.J., Vartuli, J.C., Beck, J.S.: Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359, 710–712 (1992)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinium. J. Am. Chem. Soc. 40, 1361–1403 (1918)
Leal, O., Bolivar, C., Ovalles, C., Garcia, J.J., Espidel, Y.: Reversible adsorption of carbon dioxide on amine surface-bonded silica gel. Inorg. Chim. Acta 240, 183–189 (1995)
Lee, S.-Y., Park, S.-J.: A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 23, 1–11 (2015)
Liu, X., Zhou, L., Fu, X., Sun, Y., Su, W., Zhou, Y.: Adsorption and regeneration study of the mesoporous adsorbent SBA-15 adapted to the capture/separation of CO2 and CH4. Chem. Eng. Sci. 62, 1101–1110 (2007)
Liu, S.-H., Hsiao, W.-C., Chiang, C.-C.: Synthesis of Stable tetraethylenepentamine-functionalized mesocellular silica foams for CO2 adsorption. J. Chem. 2013, 6 (2013)
Liu, Z., Teng, Y., Zhang, K., Chen, H., Yang, Y.: CO2 adsorption performance of different amine-based siliceous MCM-41 materials. J. Energy Chem. 24, 322–330 (2015)
Martínez, J.R., Palomares-Sánchez, S., Ortega-Zarzosa, G., Ruiz, F., Cchumakov, Y.: Rietveld refinement of amorphous SiO2 prepared via sol–gel method. Mater. Lett. 60, 3526–3529 (2006)
Mello, M.R., Phanon, D., Silveira, G.Q., Llewellyn, P.L., Ronconi, C.M.: Amine-modified MCM-41 mesoporous silica for carbondioxide capture. Microporous Mesoporous Mater. 143, 174–179 (2011)
Misran, H., Singh, R., Begum, S., Yarmo, M.A.: Processing of mesoporous silica materials (MCM-41) from coal fly ash. J. Mater. Process. Technol. 186, 8–13 (2007)
Monastersky, R.: A burden beyond bearing. Nature 458, 1091–1094 (2009)
Mookerjee, S.K., Niyogi, S.K.: Relation between time of gelation and concentration of added electrolyte in silicic acid sol. Bull. Cent. Glass Ceram. Res. Inst. 22, 1–5 (1975)
Musić, S., Filipović-Vinceković, N., Sekovanić, L.: Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 28(1), 89–94 (2011)
Oh, T.H.: Carbon capture and storage potential in coal-fired plant in Malaysia-A review. Renew. Sustain. Energy Rev. 14, 2697–2709 (2010)
Peng, L., Qisui, W., Xi, L., Chaocan, Z.: Investigation of the states of water and OH groups on the surface of sílica. Colloids Surf. A 334, 112–115 (2009)
Pérez-Marín, A.B., Zapata, V.M., Ortuño, J.F., Aguilar, M., Sáez, J., Lloréns, M.: Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard. Mater. B 139, 122–131 (2007)
Powell, C.E., Qiao, G.G.: Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 279, 1–49 (2006)
Prado, A.G.S., Faria, E.A., Padilha, P.M.: Aplication and chemical modification of silica-gel obtained from sand. Quim. Nova 28(3), 544–547 (2005)
Ravikovitch, P.I., Neimark, A.V.: Characterization of nanoporous materials from adsorption and desorption isotherms. Colloids Surf. A 187–188, 11–21 (2001)
Rodríguez-Estupiñán, P., Giraldo, L., Moreno-Piraján, J.C.: Calorimetric study of amino-functionalised SBA-15. J. Therm. Anal. Calorim. 121, 127–134 (2015)
Sanz, R., Calleja, G., Arencibia, A., Sanz-Perez, E.S.: CO2 capture with pore-expanded MCM-41 silica modified with amino groups by Double functionalization. Microporous Mesoporous Mater. 209, 165–171 (2015)
Shao, W., Zhang, L.Z., Li, L.X., Lee, R.L.: Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorption. 15, 497–505 (2009)
Silverstein, R.M.: Identificação espectrométrica de compostos orgânicos, 7th edn. LTC, Rio de Janeiro (2007)
Son, W.-J., Choi, J.-S., Ahn, W.-S.: Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater. 113, 31–40 (2008)
Souza, L.K.C., Pardauil, J.J.R., Zamian, J.R., Filho, G.N.R.: COSTA, C.E.F.: influence of the incorporated metal on template removal from MCM-41 type mesoporous materials. J. Therm. Anal. Calorim. 106, 355–361 (2011)
Stangeland, A.: A model for the CO2 capture potential. Int. J. Greenhouse Gas Control 1, 418–429 (2007)
Su, F., Lu, C., Chen, H.-S.: Adsorption, desorption, and thermodynamic studies of CO2 with high-amine-loaded multiwalled carbon nanotubes. Langmuir 27, 8090–8098 (2011)
Vijayalakshmi, U., Balamurugan, A., Rajeswari, S.: Synthesis and characterization of porous silica gels for biomedical applications. Trends Biomater. Artif. Organs 18(2), 101–105 (2005)
Vilarrasa-García, E., Cecilia, J.A., Moya, E.M.O., Cavalcante Jr, C.L., Azevedo, D.C.S., Rodríguez-Castellón, E.: “Low cost” pore expanded SBA-15 functionalized with amine groups applied to CO2 adsorption. Materials 8, 2495–2513 (2015)
Vunain, E., Opembe, N.N., Jalama, K., Mishra, A.K., Meijboom, R.: Thermal stability of amine-functionalized MCM-41 in different atmospheres. J. Therm. Anal. Calorim. 115, 1487–1496 (2014)
Wei, J., Liao, L., Xiao, Y., Zhang, P., Shi, Y.: Capture of carbon dioxide by amine-impregnated as synthesized MCM-41. J. Environ. Sci. 22(10), 1558–1563 (2010)
Wörmeyer, K., Alnaief, M., Smirnova, I.: Amino functionalised silica-aerogels for CO2-adsorption at low partial pressure. Adsorption 18, 163–171 (2012)
Wypych, F., Schreiner, W.H., Júnior, E.R.: Grafting of phenylarsonic and 2-nitrophenol-4-arsonic acid onto disordered silica obtained by selective leaching of brucitelike sheet from chrysotile structure. J. Colloid Interface Sci. 276, 167–173 (2004)
Xu, X., Song, C., Andresen, J.M., Miller, B.G., Scaroni, A.W.: Novel polyethylenimine modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 16, 1463–1469 (2002)
Xu, X., Song, C., Andrésen, J.M., Miller, B.G., Scaroni, A.W.: Preparation and characterization of novel CO2 ‘‘molecular basket’’ adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 62, 29–45 (2003)
Xu, X.C., Song, C.S., Miller, B.G., Scaroni, A.W.: Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous “molecular basket” adsorbent. Fuel Process. Technol. 86, 1457–1472 (2005)
Yang, H., Gong, M., Chen, Y.: Preparation of activated carbons and their adsorption properties for greenhouse gases: CH4 and CO2. J. Nat. Gas Chem. 20, 460–464 (2011)
Yang, G., Deng, Y., Ding, H., Lin, Z., Shao, Y., Wang, Y.: A facile approach to synthesize MCM-41 mesoporous materials from iron ore tailing: influence of the synthesis conditions on the structural properties. Appl. Clay Sci. 111, 61–66 (2015)
Yong, Z., Mata, V.G., Rodrigues, A.E.: Adsorption of carbon dioxide on chemically modified high surface area carbon-based adsorbents at high temperature. Adsorption 7, 41–50 (2001)
Yu, H., Xue, X., Huang, D.: Synthesis of mesoporous silica materials (MCM-41) from iron ore tailings. Mater. Res. Bull. 44, 2112–2115 (2009)
Yue, M.B., Sun, L.B., Cao, Y., Wang, Y., Wang, Z.J., Zhu, J.H.: Efficient CO2 capturer derived from as-synthesized MCM-41 modified with amine. Chem. Eur. J. 14, 3442–3451 (2008)
Zelenáka, V., Badanicová, M., Halamová, D., Cejka, J., Zukal, A., Murafa, N., Goerigk, G.: Amine-modified ordered mesoporous silica: effect of pore size on carbon dioxide capture. Chem. Eng. J. 144, 336–342 (2008)
Zhang, J., Singh, R., Webley, P.A.: Alkali and alkaline-earth cation exchanged chabazite zeolites for adsorption based CO2 capture. Microporous Mesoporous Mater. 111, 478–487 (2008)
Zhang, Z., Wang, H., Chen, X., Zhu, C., Wei, W., Sun, Y.: Chromium-based metal-organic framework/mesoporous carbon composite: synthesis, characterization and CO2 adsorption. Adsorption 21, 77–86 (2015)
Zhuravlev, L.T.: The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A 173, 1–38 (2000)
Acknowledgments
The authors thank the Federal University of Rio Grande do Norte, the Gas Technology Centre and Renewable Energy-CTGAS-ER, the researchers LABPETROL/UFRN for obtaining the adsorbents, the LABPEMOL/UFRN by XRD and calcination, the LABTAM/UFRN by SEM images and the LCE/DEMa/UFSCar by TEM images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho, L.S., Silva, E., Andrade, J.C. et al. Low-cost mesoporous adsorbents amines-impregnated for CO2 capture. Adsorption 21, 597–609 (2015). https://doi.org/10.1007/s10450-015-9710-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9710-8