Abstract
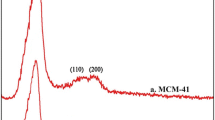
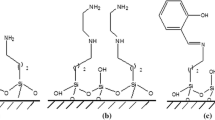
Ordered mesoporous molecular sieves are widely studied as alternative materials in areas where sorptive and catalytic applications are required. MCM-41 type mesoporous material was tested as sorbent of 2-methylbenzoic acid (MBA), an aromatic carboxylic acid selected as model molecule for adsorption studies on mesoporous silicas. Adsorption kinetic studies of MBA on MCM-41 type materials were carried out using ethanol solutions at different MBA concentrations. Experimental results followed Langmuir isotherm model showing large adsorption capacity (3.5 g/g). Two kinetic models, the pseudo first- and second-order, were selected to describe the adsorption process and to determine the best model fitting with the experimental data. Kinetic parameters for each kinetic equation were calculated and discussed. It was shown that the MBA adsorption process onto MCM-41 material could be described by the pseudo-second-order equation and that the MCM-41 performs as a suitable adsorbent material.









Similar content being viewed by others
Abbreviations
- C e :
-
Liquid-phase concentration at equilibrium
- C t :
-
Liquid-phase concentration at time t
- C 0 :
-
Initial liquid-phase concentration
- k 1 :
-
Pseudo-first-order rate constant defined in Eq. 2
- k 2 :
-
Pseudo-second-order rate constant defined in Eq. 4
- K L :
-
Langmuir constant defined in Eq.1
- q e :
-
Amount of adsorption at equilibrium
- q t :
-
Amount of adsorption at time t
- S BET :
-
BET specific surface area of adsorbent particles
- V P :
-
Total pore volume of the adsorbent
- d P :
-
Pore diameter of the adsorbent
- m/V :
-
Dosage of the adsorbent
- t :
-
Time
References
Hoffmann F, Cornelius M, Morell J, Froba M (2006) Angew Chem Int Ed 45:3216–3251
Fenolov VB, Romannikov VN, Derevyankin AYu (1999) Microporous Mesoporous Mater 28:57–72
Xia K, Ferguson RZ, Losier M, Tchoukanova N, Bruning R, Djaoued Y (2010) J Hazard Mater 183:554–564 and therein
Huang CH, Chang KP, Ou HD, Chiang YC, Wang CF (2011) Microporous Mesoporous Mater 141:102–109
Kim YH, Lee B, Choo KH, Choi SJ (2011) Microporous Mesoporous Mater 138:184–190
Shin EW, Han JS, Jang M, Min SH, Park JK, Rowell RM (2004) Environ Sci Technol 38:912–917
Bulut E, Ozacar M, Sengil IA (2008) Microporous Mesoporous Mater 115:234–246
Azizian S (2004) J Colloid Interface Sci 276:47–52
Ozacar M, Sengil IA, Turkmenler H (2008) Chem Eng J 143:32–42
Ho YS, McKay G (2003) Process Biochem 38:1047–1061
Ho YS, McKay G (1999) Process Biochem 34:451–465
Ho YS (2006) J Hazard Mater 136:681–689
Azais T, Hartmeyer G, Quignard S, Laurent G, babonneau F (2010) J Phys Chem C 114:8884–8891
Firouzi A, Atef F, Oertli AG, Stucky GD, Chmelka BF (1997) J Am Chem Soc 119(15):3596–3610
Kumar D, Schumacher K, Du Fresne HC, Grun M, Unger KK (2001) Colloids Surf A 187:109
Chenite A, Le Page Y (1995) Chem Mater 7:1015–1019
Schmidt R, Hausen EW, Stocker M, Akpiriage D, Ellestad OH (1995) J Am Chem Soc 117:4049
Brunauer S, Emmett PH, Teller E (1938) J Am Chem Soc 60:309
Barret P, Joyner LG, Halenda P (1951) J Am Chem Soc 73:373
El-Geundi MS (1991) Water Res 40:271–273
Low KS, Lee CK (1997) Bioresour Technol 121:121–125
Giles CH, MacEwan TH, Nakhwa SM, Smith D (1960) J Chem Soc Lond 56:1973–2993
Safarik I, Nymburska K, Safarikova M (1997) J Chem Technol Biotechnol 69:1
El-Khaiary MI, Malash GF, Ho YS (2010) Desalination 257:93–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aiello, D., Mirabelli, I. & Testa, F. Adsorption of 2-methylbenzoic acid onto MCM-41 mesoporous material: kinetics and equilibrium studies. J Sol-Gel Sci Technol 64, 1–8 (2012). https://doi.org/10.1007/s10971-012-2821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2821-8