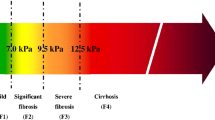
Abstract
Liver sinusoidal endothelial cells (LSECs) are the gatekeeper of liver to maintain hepatic homeostasis. They are formed into the highly specialized endothelium between vascular lumen and the space of Disse and are mechanosensitive to respond varied microenvironments. Shear stress and mechanical stretch induced by blood perfusion and substrate stiffness enhancement derived from deposition of extracellular matrix (ECM) are major mechanical stimuli that surround LSECs. This review introduces how LSECs respond to the external forces in both physiological and pathological cases and what is the interplay of LSECs with other hepatic cells. Molecular mechanisms that potentiate LSECs mechanotransduction are also discussed.
Graphic abstract






Similar content being viewed by others
Change history
19 March 2021
The original version is updated due to equation 1 has been incorrectly appeared in PDF
Abbreviations
- AFM:
-
Atomic force microscopy
- ARFI:
-
Acoustic radiation force impulse imaging
- BMP:
-
Bone morphogenic protein
- BMSC:
-
Bone marrow stromal cell
- CFD:
-
Computational fluid dynamics
- CH:
-
Congestive hepatopathy
- CT:
-
Computed tomography
- Dnmt3b:
-
DNA methyltransferase 3b
- ECM:
-
Extracellular matrix
- eNOS:
-
Endothelial nitric oxide synthase
- EVG:
-
Elastica van Gieson
- F-actin:
-
Filamentous actin
- HCV:
-
Hepatitis C virus
- HGF:
-
Hepatocyte growth factor
- Hh:
-
Hedgehog
- HLH:
-
Helix–loop–helix
- HSC:
-
Hepatic stellate cell
- HUVEC:
-
Human umbilical vein endothelial cell
- IAA:
-
Iodoacetic acid
- Id1:
-
Inhibitor of DNA binding 1
- Ihh:
-
Indian hedgehog
- I/R:
-
Ischemia-reperfusion
- KLF2:
-
Kruppel-like factor 2
- LOX:
-
Lysyl oxidase
- LPA:
-
Lysophosphatidic acid
- LSEC:
-
Liver sinusoidal endothelial cell
- LXRα:
-
Liver X receptor α
- MEC:
-
Mammary epithelial cell
- MMP9:
-
Matrix metalloproteinase-9
- MRE:
-
Magnetic resonance elastography
- MSC:
-
Mesenchymal stem cell
- NAFLD:
-
Nonalcoholic fatty liver disease
- NECD:
-
Notch extracellular domain
- NETs:
-
Neutrophil extracellular traps
- NICD:
-
Notch intracellular domain
- NO:
-
Nitric oxide
- NRP1:
-
Neuropilin-1
- PH:
-
Partial hepatectomy
- pIVCL:
-
Partial inferior vena cava ligation
- PVE:
-
Portal vein embolization
- ROS:
-
Reactive oxygen species
- RPM:
-
Revolutions per minute
- Shh:
-
Sonic hedgehog
- SMC:
-
Smooth muscle cell
- TG:
-
Transglutaminase
- TM:
-
Thrombomodulin
- UTE:
-
Ultrasound-based technique of transient elastography
- VEGFR2:
-
Vascular endothelial cell growth factor receptor 2
- VEGFR3:
-
Vascular endothelial cell growth factor receptor 3
- YAP:
-
Yes-associated protein
References
Poisson, J., Lemoinne, S., Boulanger, C., et al.: Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 66(1), 212–227 (2017)
Maslak, E., Gregorius, A., Chlopicki, S.: Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacol. Rep. 67(4), 689–694 (2015)
DeLeve, L.D.: Vascular liver disease and the liver sinusoidal endothelial cell. In: DeLeve, L.D., Garcia-Tsao, G. (eds.) Vascular Liver Disease: Mechanisms and Management. Springer, New York (2011)
Wisse, E.: An electron microscopic study of fenestrated endothelial lining of rat liver sinusoids. J. Ultrastruct. Res. 31(1), 125–150 (1970)
Braet, F., Wisse, E.: Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 1(1), 1 (2002)
Li, P.W., Zhou, J., Li, W., et al.: Characterizing liver sinusoidal endothelial cell fenestrae on soft substrates upon AFM imaging and deep learning. Biochim. Biophys. Acta Gen. Subj. 1864(12), 129702 (2020).
Wisse, E., De Zanger, R.B., Charels, K., et al.: The liver sieve—considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 5(4), 683–692 (1985)
Shetty, S., Lalor, P.F., Adams, D.H.: Liver sinusoidal endothelial cells—gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15(9), 555–567 (2018)
Marrone, G., Shah, V.H., Gracia-Sancho, J.: Sinusoidal communication in liver fibrosis and regeneration. J. Hepatol. 65(3), 608–617 (2016)
Hammoutene, A., Rautou, P.: Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J.. Hepatol. 70(6), 1278–1291 (2019)
Vollmar, B., Menger, M.D.: The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 89(4), 1269–1339 (2009)
Schaffner, F., Popper, H.: Capillarization of hepatic sinusoids in man. Gastroenterology. 44(3), 239–242 (1963)
Urashima, S., Tsutsumi, M., Nakase, K., et al.: Studies on capillarization of the hepatic sinusoids in alcoholic liver-disease. Alcohol Alcohol Suppl. 28(1B), 77–84 (1993)
Xu, B., Broome, U., Uzunel, M., et al.: Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am. J. Pathol. 163(4), 1275–1289 (2003)
DeLeve, L.D.: Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 61(5), 1740–1746 (2015)
You, Z.F., Zhou, L., Li, W.J., et al.: Mechanical microenvironment as a key cellular regulator in the liver. Acta Mech. Sin. 35(2), 289–298 (2019)
Eipel, C., Abshagen, K., Vollmar, B.: Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J. Gastroenterol. 16(48), 6046–6057 (2010)
Grande Nakazato, P.C., Victorino, J.P., Fina, C.F., et al.: Liver ischemia and reperfusion injury. Pathophysiology and new horizons in preconditioning and therapy. Acta Cir. Bras. 33(8), 723–735 (2018)
Golse, N., Bucur, P.O., Adam, R., et al.: New paradigms in post-hepatectomy liver failure. J. Gastrointest. Surg. 17(3), 593–605 (2013)
Lorenz, L., Axnick, J., Buschmann, T., et al.: Mechanosensing by beta 1 integrin induces angiocrine signals for liver growth and survival. Nature 562(7725), 128–132 (2018)
DeLeve, L.D., Wang, X.D., Guo, Y.M.: Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48(3), 920–930 (2008)
Garcia-Pagan, J.C., Gracia-Sancho, J., Bosch, J.: Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J. Hepatol. 57(2), 458–461 (2012)
Thabut, D., Shah, V.: Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J. Hepatol. 53(5), 976–980 (2010)
Georges, P.C., Hui, J.J., Gombos, Z., et al.: Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 293(6), G1147–G1154 (2007)
Ajmera, V.H., Liu, A., Singh, S., et al.: Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology 71(3), 849–860 (2020)
Xing, X., Yan, Y.L., Shen, Y., et al.: Liver fibrosis with two-dimensional shear-wave elastography in patients with autoimmune hepatitis. Expert Rev. Gastroenterol. Hepatol. 14(7), 631–638 (2020)
Higuchi, M., Tamaki, N., Kurosaki, M., et al: Changes of liver stiffness measured by magnetic resonance elastography during direct-acting antivirals treatment in patients with chronic hepatitis C. J. Med. Virol. Online ahead of print
Medrano, L.M., Garcia-Broncano, P., Berenguer, J., et al.: Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/hepatitis C virus-coinfected patients. AIDS 32(9), 1095–1105 (2018)
Ko, B.J., Kim, Y.S., Kim, S.G., et al.: Relationship between 25-hydroxyvitamin d levels and liver fibrosis as assessed by transient elastography in patients with chronic liver disease. Gut Liver. 10(5), 818–825 (2016)
Davies, P.F.: Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75(3), 519–560 (1995)
Ballermann, B.J., Dardik, A., Eng, E., et al.: Shear stress and the endothelium. Kidney Int. 54, S100–S108 (1998)
Johnson, F.P.: The development of the lobule of the pig’s liver. Am. J. Anat. 25(3), 299–331 (1919)
Deal, C.P., Green, H.D.: Comparison of changes in mesenteric resistance following splanchnic nerve stimulation with responses to epinephrine and norepinephrine. Circ. Res. 4(1), 38–44 (1956)
Wen, B., Liang, J., Deng, X., et al.: Effect of fluid shear stress on portal vein remodeling in a rat model of portal hypertension. Gastroenterol. Res. Pract. 41, 1–7 (2015)
Aghasafari, P., Pidaparti, R.: Influence of tidal-volume setting, emphysema and ARDS on human alveolar sacs mechanics. Acta Mech. Sin. 34(5), 983–993 (2018)
Liu, Z.M., Zhao, S.W., Li, Y.J., et al.: Influence of coronary bifurcation angle on atherosclerosis. Acta Mech. Sin. 35(6), 1269–1278 (2019)
Debbaut, C., Vierendeels, J., Casteleyn, C., et al.: Perfusion characteristics of the human hepatic microcirculation based on three-dimensional reconstructions and computational fluid dynamic analysis. J. Biomech. Eng. 134(1), 011003 (2012)
Wei, W., Pu, Y.S., Wang, X.K., et al.: Wall shear stress in portal vein of cirrhotic patients with portal hypertension. World J. Gastroenterol. 23(18), 3279–3286 (2017)
Peeters, G., Debbaut, C., Cornillie, P., et al.: A multilevel modeling framework to study hepatic perfusion characteristics in case of liver cirrhosis. J. Biomech. Eng. 137(5), 051007 (2015)
Hu, J.R., Lu, S.Q., Feng, S.L., et al.: Flow dynamics analyses of pathophysiological liver lobules using porous media theory. Acta Mech. Sin. 33(4), 823–832 (2017)
Fernandez, M.: Molecular pathophysiology of portal hypertension. Hepatology 61(4), 1406–1415 (2015)
Shah, V., Haddad, F.G., Garcia-Cardena, G., et al.: Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Invest. 100(11), 2923–2930 (1997)
Gracia-Sancho, J., Russo, L., Garcia-Caldero, H., et al.: Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut 60(4), 517–524 (2011)
Conway, E.M.: Thrombomodulin and its role in inflammation. Semin. Immunopathol. 34(1), 107–125 (2012)
Marrone, G., Russo, L., Rosado, E., et al.: The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J. Hepatol. 58(1), 98–103 (2013)
Garcea, G., Maddern, G.J.: Liver failure after major hepatic resection. J. Hepato-Biliary Pancreatic Surg. 16(2), 145–155 (2009)
Schoen, J.M., Wang, H.H., Minuk, G.Y., et al.: Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide 5(5), 453–464 (2001)
Braet, F., Shleper, M., Paizi, M., et al.: Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp. Hepatol. 3(1), 7 (2004)
Kim, H.J., Chung, H., Yoo, Y.G., et al.: Inhibitor of DNA binding 1 activates vascular endothelial growth factor through enhancing the stability and activity of hypoxia-inducible factor-1 alpha. Mol. Cancer Res. 5(4), 321–329 (2007)
Wang, H., Yu, Y., Guo, R.W., et al.: Inhibitor of DNA binding-1 promotes the migration and proliferation of endothelial progenitor cells in vitro. Mol. Cell. Biochem. 335(1–2), 19–27 (2010)
Ding, B.S., Nolan, D.J., Butler, J.M., et al.: Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468(7321), 310–315 (2010)
Yamanaka, K., Hatano, E., Narita, M., et al.: Olprinone attenuates excessive shear stress through up-regulation of endothelial nitric oxide synthase in a rat excessive hepatectomy model. Liver Transplant. 17(1), 60–69 (2011)
Morsiani, E., Mazzoni, M., Aleotti, A., et al.: Increased sinusoidal wall permeability and liver fatty change after two-thirds hepatectomy: an ultrastructural study in the rat. Hepatology 21(2), 539–544 (1995)
Torii, T., Miyazawa, M., Koyama, I.: Effect of continuous application of shear stress on liver tissue: continuous application of appropriate shear stress has advantage in protection of liver tissue. Transpl. Proc. 37(10), 4575–4578 (2005)
Asencio, J.M., Garcia-Sabrido, J.L., Lopez-Baena, J.A., et al.: Preconditioning by portal vein embolization modulates hepatic hemodynamics and improves liver function in pigs with extended hepatectomy. Surgery 161(6), 1489–1501 (2017)
Asakura, T., Ohkohchi, N., Orii, T., et al.: Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl. Int. 16(6), 376–382 (2003)
Lentsch, A.B., Kato, A., Yoshidome, H., et al.: Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 32(2), 169–173 (2000)
Peralta, C., Jimenez-Castro, M.B., Gracia-Sancho, J.: Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 59(5), 1094–1106 (2013)
Huet, P.M., Nagaoka, M.R., Desbiens, G., et al.: Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. Hepatology 39(4), 1110–1119 (2004)
Garcia-Valdecasas, J.C., Fondevila, C.: In-vivo normothermic recirculation: an update. Curr. Opin. Org. Transpl. 15(2), 173–176 (2010)
Xue, S., He, W.Y., Zeng, X.P., et al.: Hypothermic machine perfusion attenuates ischemia/reperfusion injury against rat livers donated after cardiac death by activating the Keap1/Nrf2-ARE signaling pathway. Mol. Med. Rep. 18(1), 815–826 (2018)
Chatterjee, S., Nieman, G.F., Christie, J.D., et al.: Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am. J. Physiol. Lung Cell. Mol. Physiol. 307(9), L668–L680 (2014)
Hammoutene, A., Biquard, L., Lasselin, J., et al.: A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol 72(3), 528–538 (2020)
Boteon, Y.L., Laing, R., Mergental, H., et al.: Mechanisms of autophagy activation in endothelial cell and their targeting during normothermic machine liver perfusion. World J. Gastroenterol. 23(48), 8443–8451 (2017)
Noh, J.K., Jung, J.G., Jang, E.M., et al.: Live cell-imaging perfusion culture system of liver sinusoidal endothelial cells to mimic stem cell engraftment in liver. Transpl. Proc. 44(4), 1116–1119 (2012)
Shetty, S., Weston, C.J., Oo, Y.H., et al.: Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol. 186(7), 4147–4155 (2011)
Busse, R., Fleming, I.: Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J. Vasc. Res. 35(2), 73–84 (1998)
Rabbany, S.Y., Ding, B.S., Larroche, C., et al.: Mechanosensory pathways in angiocrine mediated tissue regeneration. In: Gefen, A., Aviv, R. (eds.) Studies in Mechanobiology, Tissue Engineering and Biomaterials. Springer, Berlin, Heidelberg, New York (2013)
Hilscher, M.B., Sehrawat, T., Arab, J.P., et al.: Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension. Gastroenterology. 157(1), 193–209 (2019)
Wanless, I.R., Liu, J.J., Butany, J.: Role of thrombosis in the pathogenesis of congestive hepatic-fibrosis (cardiac cirrhosis). Hepatology. 21(5), 1232–1237 (1995)
Soydemir, S., Comella, O., Abdelmottaleb, D., et al.: Does mechanocrine signaling by liver sinusoidal endothelial cells offer new opportunities for the development of anti-fibrotics? Front. Med. 6, 312 (2020)
Kawai, M., Naruse, K., Komatsu, S., et al.: Mechanical stress-dependent secretion of interleukin 6 by endothelial cells after portal vein embolization: clinical and experimental studies. J. Hepatol. 37(2), 240–246 (2002)
Cressman, D.E., Greenbaum, L.E., DeAngelis, R.A., et al.: Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274(5291), 1379–1383 (1996)
Sakamoto, T., Liu, Z.J., Murase, N., et al.: Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 29(2), 403–411 (1999)
Bohm, F., Kohler, U.A., Speicher, T., et al.: Regulation of liver regeneration by growth factors and cytokines. EMBO Mol. Med. 2(8), 294–305 (2010)
Olle, E.W., Ren, X.D., McClintock, S.D., et al.: Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology 44(3), 540–549 (2006)
Handorf, A.M., Zhou, Y.X., Halanski, M.A., et al.: Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11(1), 1–15 (2015)
Sandrin, L., Fourquet, B., Hasquenoph, J.M., et al.: Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29(12), 1705–1713 (2003)
Mueller, S., Sandrin, L.: Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat. Med. 2, 49–67 (2010)
Palmeri, M.L., Wang, M.H., Dahl, J.J., et al.: Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med. Biol. 34(4), 546–558 (2008)
Wegner, M., Iskender, E., Azzarok, A., et al.: Comparison of acoustic radiation force impulse imaging with the convex probe 6C1 and linear probe 9L4. Medicine (Baltimore). 99(16), e19701 (2020)
Dahl, J.J., Pinton, G.F., Palmeri, M.L., et al.: A parallel tracking method for acoustic radiation force impulse imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 54(2), 301–312 (2007)
Palmeri, M.L., Sharma, A.C., Bouchard, R.R., et al.: A finite-element method model of soft tissue response to impulsive acoustic radiation force. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 52(10), 1699–1712 (2005)
Muthupillai, R., Lomas, D.J., Rossman, P.J., et al.: Magnetic-resonance elastography by direct visualization of propagating acoustic strain waves. Science 269(5232), 1854–1857 (1995)
Venkatesh, S.K., Yin, M., Ehman, R.L.: Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J. Magn. Reson. Imaging 37, 544–555 (2013)
Taouli, B., Ehman, R.L., Reeder, S.B.: Advanced MRI methods for assessment of chronic liver disease. AJR Am. J .Roentgenol. 193(1), 14–27 (2009)
Gang, Z., Qi, Q., Jing, C., et al.: Measuring microenvironment mechanical stress of rat liver during diethylnitrosamine induced hepatocarcinogenesis by atomic force microscope. Microsc. Res. Tech. 72(9), 672–678 (2009)
Hu, J.R., Huang, D.D., Zhang, Y., et al.: Global mapping of live cell mechanical features using PeakForce QNM AFM. Biophys. Rep. 6(1), 9–18 (2020)
Lin, D.C., Dimitriadis, E.K., Horkay, F.: Advances in the mechanical characterization of soft materials by nanoindentation. Recent Res. Dev. Biophys. 5, 333–370 (2006)
Desai, S.S., Tung, J.C., Zhou, V.X., et al.: Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology 64(1), 261–275 (2016)
Butt, H.J., Jaschke, M., Ducker, W.: Measuring surface forces in aqueous-electrolyte solution with the atomic-force microscope. Bioelectrochem. Bioenerget. 38(1), 191–201 (1995)
Efremov, Y.M., Okajima, T., Raman, A.: Measuring viscoelasticity of soft biological samples using atomic force microscopy. Soft Matter 16(1), 64–81 (2020)
Bastard, C., Bosisio, M.R., Chabert, M., et al.: Transient micro-elastography: a novel non-invasive approach to measure liver stiffness in mice. World J. Gastroenterol. 17(8), 968–975 (2011)
Hoodeshenas, S., Yin, M., Venkatesh, S.K.: Magnetic resonance elastography of liver: current update. Top. Magn. Reson. Imaging 27(5), 319–333 (2018)
Baiocchini, A., Montaldo, C., Conigliaro, A., et al.: Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS ONE 11(3), e0151736 (2016)
Abe, T., Hashiguchi, A., Yamazaki, K., et al.: Quantification of collagen and elastic fibers using whole-slide images of liver biopsy specimens. Patho.l Int. 63(6), 305–310 (2013)
Kolacna, L., Bakesova, J., Varga, F., et al.: Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 56(Suppl 1), S51–S60 (2007)
Barry-Hamilton, V., Spangler, R., Marshall, D., et al.: Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16(9), 1009–1017 (2010)
Ford, A.J., Jain, G., Rajagopalan, P.: Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomater. 24, 220–227 (2015)
Juin, A., Planus, E., Guillemot, F., et al.: Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biol. Cell 105(1), 46–57 (2013)
Liu, L.W., You, Z.F., Yu, H.S., et al.: Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat. Mater. 16(12), 1252–1261 (2017)
Bok, J., Zenczak, C., Hwang, C.H., et al.: Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. USA 110(34), 13869–13874 (2013)
Masai, I., Yamaguchi, M., Tonou-Fujimori, N., et al.: The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation. Development 132(7), 1539–1553 (2005)
Abramyan, J.: Hedgehog signaling and embryonic craniofacial disorders. J. Dev. Biol. 7(2), 9 (2019)
Binder, M., Chmielarz, P., McKinnon, P.J., et al.: Functionally distinctive Ptch receptors establish multimodal Hedgehog signaling in the tooth epithelial stem cell niche. Stem Cells 37(9), 1238–1248 (2019)
Wang, C.D., Shan, S.Z., Wang, C.L., et al.: Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp. Cell. Res. 352(2), 346–356 (2017)
Wu, Q.Q., Zhang, Y., Chen, Q.: Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J. Biol. Chem. 276(38), 35290–35296 (2001)
Morrow, D., Sweeney, C., Birney, Y.A., et al.: Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am. J. Physiol. Cell Physiol. 292(1), C488–C496 (2007)
Hoey, D.A., Downs, M.E., Jacobs, C.R.: The mechanics of the primary cilium: an intricate structure with complex function. J. Biomech. 45(1), 17–26 (2012)
Corbit, K.C., Aanstad, P., Singla, V., et al.: Vertebrate Smoothened functions at the primary cilium. Nature 437(7061), 1018–1021 (2005)
Kim, J., Kato, M., Beachy, P.A.: Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl. Acad. Sci. USA 106(51), 21666–21671 (2009)
Xie, G.H., Choi, S.S., Syn, W.K., et al.: Hedgehog signalling regulates liver sinusoidal endothelial cell capillarization. Gut 62(2), 299–309 (2013)
Pereira, T.D., Witek, R.P., Syn, W.K., et al.: Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab. Invest. 90(12), 1690–1703 (2010)
Witek, R.P., Yang, L., Liu, R.S., et al.: Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 136(1), 320–330 (2009)
Xing, Y., Zhao, T.T., Gao, X.Y., et al.: Liver X receptor alpha is essential for the capillarization of liver sinusoidal endothelial cells in liver injury. Sci. Rep. 6, 11 (2016)
Yang, X., Wang, Z.M., Kai, J., et al.: Curcumol attenuates liver sinusoidal endothelial cell angiogenesis via regulating Glis-PROX1-HIF-1 alpha in liver fibrosis. Cell Prolif. 53(3), 134–144 (2020)
Weinmaster, G., Fischer, J.A.: Notch ligand ubiquitylation: what is it good for? Dev. Cell. 21(1), 134–44 (2011)
Henrique, D., Schweisguth, F.: Mechanisms of Notch signaling: a simple logic deployed in time and space. Development 146(3), dev172148 (2019).
D’Souza, B., Miyamoto, A., Weinmaster, G.: The many facets of Notch ligands. Oncogene 27(38), 5148–5167 (2008)
Kopan, R.: Notch Signaling. Cold Spring Harb. Perspect. Biol. 4(10), a011213 (2012).
Shen, W., Sun, J.J.: Different modes of Notch activation and strength regulation in the spermathecal secretory lineage. Development 147(3), dev184390 (2020).
Meloty-Kapella, L., Shergill, B., Kuon, J., et al.: Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev. Cell 22(6), 1299–1312 (2012)
Mack, J.J., Mosqueiro, T.S., Archer, B.J., et al.: NOTCH1 is a mechanosensor in adult arteries. Nat. Commun. 8(1), 1620 (2017)
Loomes, K.M., Taichman, D.B., Glover, C.L., et al.: Characterization of Notch receptor expression in the developing mammalian heart and liver. Am. J. Med. Genet. 112(2), 181–189 (2002)
Cuervo, H., Nielsen, C.M., Simonetto, D.A., et al.: Endothelial Notch signaling is essential to prevent hepatic vascular malformations in mice. Hepatology 64(4), 1302–1316 (2016)
Duan, J.-L., Ruan, B., Yan, X.-C., et al.: Endothelial Notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice. Hepatology 68(2), 677–690 (2018)
Chen, L.Y., Gu, T.Y., Li, B.H.: Delta-like ligand 4/DLL4 regulates the capillarization of liver sinusoidal endothelial cell and liver fibrogenesis. Biochim. Biophys. Acta Mol. Cell. Res. 1866(10), 1663–1675 (2019)
Bai, H.B., Zhang, N.L., Xu, Y., et al.: Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 56(3), 1097–1107 (2012)
Zhang, C.X., Bian, M.L., Chen, X.R., et al.: Oroxylin A prevents angiogenesis of LSECs in liver fibrosis via inhibition of YAP/HIF-1 signaling. J. Cell. Biochem. 119(2), 2258–2268 (2018)
Zheng, Y.G., Pan, D.J.: The Hippo signaling pathway in development and disease. Dev. Cell 50(3), 264–282 (2019)
Dupont, S., Morsut, L., Aragona, M., et al.: Role of YAP/TAZ in mechanotransduction. Nature 474(7350), 179–183 (2011)
Totaro, A., Panciera, T., Piccolo, S.: YAP/TAZ upstream signals and downstream responses. Nat. Cell. Biol. 20(8), 888–899 (2018)
Nakajima, H., Yamamoto, K., Agarwala, S., et al.: Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev. Cell 40(6), 523–536 (2017)
Neto, F., Klaus-Bergmann, A., Ong, Y.T., et al.: YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. eLife 7, e31037 (2018).
Watanabe, N., Tohyama, K., Yamashiro, S.: Mechanostress resistance involving formin homology proteins: G- and F-actin homeostasis-driven filament nucleation and helical polymerization-mediated actin polymer stabilization. Biochem. Biophys. Res. Commun. 506(2), 323–329 (2018)
Wang, Y.Z., Qian, J.: Buckling of filamentous actin bundles in filopodial protrusions. Acta Mech. Sin. 35(2), 365–375 (2019)
Kadzik, R.S., Homa, K.E., Kovar, D.R.: F-actin cytoskeleton network self-organization through competition and cooperation. Annu. Rev. Cell. Dev. Biol. 36, 35–60 (2020)
Merino, F., Pospich, S., Raunser, S.: Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin. Cell. Dev. Biol. 102, 51–64 (2020)
Sun, X.Y., Phua, D.Y.Z., Axiotakis, L.J., et al.: Mechanosensing through direct binding of tensed f-actin by LIM domains. Dev. Cell 55(4), 468–482 (2020)
Stricker, J., Falzone, T., Gardel, M.L.: Mechanics of the F-actin cytoskeleton. J. Biomech. 43(1), 9–14 (2010)
Monkemoller, V., Oie, C., Hubner, W., et al.: Multimodal super-resolution optical microscopy visualizes the close connection between membrane and the cytoskeleton in liver sinusoidal endothelial cell fenestrations. Sci. Rep. 5, 16279 (2015).
Zapotoczny, B., Szafranska, K., Owczarczyk, K., et al.: Atomic force microscopy reveals the dynamic morphology of fenestrations in live liver sinusoidal endothelial cells. Sci. Rep. 7(1), 7994 (2017)
Zapotoczny, B., Braet, F., Kus, E., et al.: Actin-spectrin scaffold supports open fenestrae in liver sinusoidal endothelial cells. Traffic 20(12), 932–942 (2019)
Ridley, A.J., Hall, A.: The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth-factors. Cell 70(3), 389–399 (1992)
Yokomori, H., Yoshimura, K., Funakoshi, S., et al.: Rho modulates hepatic sinusoidal endothelial fenestrae via regulation of the actin cytoskeleton in rat endothelial cells. Lab. Invest. 84(7), 857–864 (2004)
Beijert, I., Mert, S., Huang, V., et al.: Endothelial dysfunction in steatotic human donor livers: a pilot study of the underlying mechanism during subnormothermic machine perfusion. Transpl. Direct 4(5), e345 (2018)
Du, Y., Li, N., Yang, H., et al.: Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab. Chip 17(5), 782–794 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 91642203, 31627804, and 31870930), the Scientific Instrument Developing Project, Strategic Priority Research Program and Frontier Science Key Project of Chinese Academy of Sciences (Grants GJJSTU20190005, QYZDJ-SSW-JSC018 and XDB22040101).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Executive Editor: Xi-Qiao Feng
Rights and permissions
About this article
Cite this article
Shu, X., Li, N., Wu, Y. et al. Mechanotransduction of liver sinusoidal endothelial cells under varied mechanical stimuli. Acta Mech. Sin. 37, 201–217 (2021). https://doi.org/10.1007/s10409-021-01057-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-021-01057-3