Abstract
Mixed-species forests can provide higher levels of ecosystem functions and services and can be more resistant and resilient in the face of global change. While many studies focus on the growth and yield of mixed forests, fewer have examined the underlying processes. Inter- or intra-specific differences and interactions influence tree- and stand-level light absorption by determining the vertical structure of stratified canopies, stand density, leaf area index, and the size or allometry of trees. While canopy light absorption is a very important process, it is difficult to quantify it for individual species within a mixture and is rarely examined. A detailed tree-level model (MAESTRA) was used in combination with measurements of tree sizes and stand structures to examine effects of mixing on absorbed photosynthetically active radiation (APAR) in 41–63-year-old stands of Pseudotsuga menziesii and Fagus sylvatica at three sites in Bavaria, Germany. The effects of initial stand density on APAR were analysed in 46-year-old P. menziesii stands of a spacing experiment at two sites. At the tree level, mixing increased mean height and leaf area, growth (185% higher) and APAR (85% higher) of P. menziesii at all sites. Mean tree heights and crown sizes of F. sylvatica were larger in mixtures, while recent growth rates and APAR were not significantly different to monocultures. Planting density did not influence mean tree variables (e.g. height, leaf area, crown volume), because any initial spacing effects had been gradually removed by thinning across all treatments. At the stand level, there were no differences in growth, basal area or in the annual growth per annual APAR (light use efficiency, LUE) between monocultures and mixtures. The highest APAR values were observed in P. menziesii monocultures, while the lowest APAR values were observed in F. sylvatica monocultures. While mixing these species may not increase stand-level growth during later phases of development, mixing accelerated initial growth of individual trees and reduced the time to reach target diameters, which are both important aspects in adapting forests to global change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mixed-species forests have been promoted as a major pathway of forest management to mitigate the effects of global change, to increase the productivity of forests, and to enable a sustainable provision of forest ecosystem services in general because they can be more resistant and resilient towards climate change than monocultures (Zhang et al. 2012; Grossiord et al. 2014; Forrester and Bauhus 2016; Barbeito et al. 2017; Jactel et al. 2017; Ammer 2019; Forrester 2019; Messier et al. 2022).
Complementarity is a reduction in competition due to interspecific differences in resource use, or a facilitative effect, where plants interact in such a way that at least one species positively influences another (Vandermeer 1989). Mixing tree species can lead to complementarity through physiological, phenological, and morphological differences (within and between species) that result from interspecific interactions in mixed-species forests.
Light dynamics in mixed-species forests are directly driven by the spatiotemporal complementary use of canopy space. Mixing tree species with contrasting phenology (e.g. deciduous vs. evergreen) or differences in crown shape, crown size, crown leaf area density, as well as different tolerances to shade, influences light interception, which influence tree growth (Ishii and Asano 2010; Niinemets 2010; Sapijanskas et al. 2014; Forrester et al. 2018; Williams et al. 2021). For instance, a well-studied model of mixed planted forests is mixtures of Eucalyptus and nitrogen (N)-fixing species, where growth, APAR, and LUE were all greater in mixtures than in monocultures (Bauhus et al. 2004; Binkley et al. 1992; Forrester et al. 2012a, b). The drivers behind increases in APAR were greater crown sizes, higher leaf area index (LAI), and canopy stratification resulting in a reduction of light competition for one or both species (Bauhus et al. 2004; Binkley et al. 1992; Forrester et al. 2012a, b). Increases in LUE were associated with higher rates of photosynthesis (Forrester et al. 2012a, b), which could have been caused by higher availability or use of other resources such as nitrogen, phosphorous, and water, and shifts in carbon allocation from below ground to above ground (Forrester et al. 2005, 2006, 2010). In contrast, on sites where Eucalyptus outcompeted the N-fixing species, Eucalyptus monocultures had the highest biomass growth and LUE (le Maire et al. 2013). APAR was intermediate between the mixtures (50% mix of each species) and monocultures of the N-fixing species, which had the lowest growth and APAR. In these stands, Eucalyptus trees were much larger than the N-fixing species (le Maire et al. 2013). The APAR of individual Eucalyptus was about 169% greater in mixtures than in monocultures, while that of the N-fixing species in mixtures was only about 68% of that in monocultures (le Maire et al. 2013).
Differences in stand density and thinning can also influence APAR and LUE. Individual tree growth and APAR of Eucalyptus species increased following thinning in monocultures, and in some cases LUE also increased (e.g. Forrester et al. 2013; West and Osler 1995). Similarly observations were made for mixtures where density effects led to an average 14% increase in APAR for mixed stands of Pinus sylvestris and Fagus sylvatica (Forrester et al. 2018).
More recent studies about the contribution of light-related interactions or structural characteristics on APAR and LUE found that in addition to the structural and spatial characteristics (e.g. crown architecture, positioning of the different trees, leaf angle distributions, etc.), phenological differences between species can lead to complementary use of light (Forrester and Albrecht 2014; Sapijanskas et al. 2014; Forrester et al. 2018, 2019). However, the relative contribution of these structural and phenological factors to canopy APAR depends on the species and site characteristics, as well as how silvicultural interventions influence stand structure. Therefore, the relative contribution is difficult to predict given the small number of studies. A better understanding could aid the design of silvicultural interventions that further improve APAR, LUE, and hence forest growth and tree vitality. In addition, a better understanding of light-related tree species interactions in relation to canopy structure can improve the modelling of photosynthesis and productivity in process-based forest growth models (Pretzsch et al. 2015; Bravo et al. 2019) and Earth system models (Braghiere et al. 2019).
Two tree species that are currently of great interest economically and ecologically in Central Europe are Fagus sylvatica and Pseudotsuga menziesii. The native F. sylvatica has a broad ecological amplitude, a high growth performance, abundant natural regeneration, a high degree of shade tolerance and produces dense canopies (Brumme and Khanna 2009). Owing to these characteristics, F. sylvatica is a very competitive and dominant tree species that produces almost monospecific stands in most situations where site conditions are not extreme (Leuschner and Ellenberg 2017). P. menziesii, which is native to western North America, was introduced to Western and Central Europe, where it has become an important tree species due to its adaptability, wood quality, and high productivity (Kleinschmit and Bastien 1992). It can have a higher drought tolerance compared to European conifer species (Eilmann and Rigling 2012; Vitali et al. 2017) and is therefore viewed as an alternative to the most widely cultivated conifer Picea abies, which is experiencing a massive reduction in its cultivated area owing to its susceptibility to drought, wind storms, and bark beetles (e.g. Mezei et al. 2017). The mixing of these two species provides several advantages. Mixing reduces potential risks associated with the loss of a species owing to new pests or pathogens. Mixing an introduced tree species with a native species also buffers any potential negative effects the species may have on native biodiversity at the stand scale (Bauhus et al. 2017). In addition, mixing the shade-intolerant P. menziesii with the very shade-tolerant F. sylvatica prevents the spread of the non-native species through natural regeneration (Bindewald et al. 2021). From a forest growth perspective, P. menziesii can benefit from mixing with F. sylvatica in terms of drought stress release and time of growth recovery after drought events, and can also grow faster when mixed with F. sylvatica (Thurm et al. 2016). This was attributed to the height stratification of these two species and complementary crown architectures (Thurm and Pretzsch 2016; Thurm et al. 2016), which may lead to a higher stand-level light interception. Therefore, the objective of this study was to quantify the light absorption and light use efficiency of F. sylvatica and P. menziesii stands, and how these are influenced by the crown and canopy architecture. We also took advantage of a nearby stand density trial to examine whether differences in stand density (at a given time) can have comparable effects on light absorption of P. menziesii compared with mixtures. With measurements of tree dimensions, crown architecture, and stand structure, as well as the establishment of allometric equations, we tested the hypotheses:
1) Increases in APAR and LUE are both responsible for complementary effects on tree growth in mixtures of Fagus sylvatica and Pseudotsuga menziesii.
2) Intra-specific differences in crown architecture (in addition to interspecific differences) contribute to increases in APAR and LUE in mixtures.
3) Thinning or low stand densities increase individual tree growth of Pseudotsuga menziesii because they increase tree APAR, but only influence LUE on sites where thinning or low density also increases the availability of soil resources (e.g. not on sites that are nutrient-rich and moist).
Methods
Site description
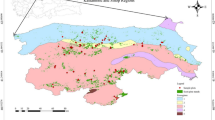
The study sites were located in Bavaria, Germany (Table 1, Fig. 1). Mixing effects were examined at three sites (Ensdorf/Hirschwald; Gramschatzer Wald; Walkertshofen), while the effects of planting density and thinning on P. menziesii were examined at two additional sites (Ansbach; Heigenbrücken).
At each site of the mixture experiment, there were three plots, forming a “triplet”, consisting of a F. sylvatica and P. menziesii mixture and their respective monocultures. The plot size of the monocultures ranged from 170 m2 to 500 m2 and the mixtures from 725 m2—1115 m2. Within each triplet, the site conditions were as similar as possible, and the stands had not been thinned for at least a decade. All plots were close to even-aged (mean age 54 years) and had been regenerated conventionally through a mix of planting and natural regeneration. Plots had not been established according to a strict experimental design with fixed species proportions and spatial patterns.
To examine the effects of planting density and thinning on P. menziesii, two similarly designed experiments were used. The Heigenbrücken experiment contained 22 plots and the experiment in Ansbach contained 21 plots. All plots had an area of 40 × 40 m2 including a 30 × 30 m2 core area and a five metres wide buffer strip. The entire site was surrounded by a 10 to 20 m wide buffer zone. The planting density experiments were planted in May 1973 using three-year-old P. menziesii with 1000, 2000 or 4000 trees per ha as the initial planting density treatments. The experimental plots were thinned between planting and the time of fieldwork for this study such that an average of 33 trees per plot remained, regardless of initial planting density. Thus, the initial density of 1000 trees ha−1 corresponds to a light thinning treatment, 2000 to a medium, and 4000 to a heavily thinning schedule (Nickel and Rais 2013, 2015). At each of the sites, two heavily thinned, two moderately thinned, and two lightly thinned plots were sampled.
The methods described below were applied to all plots, with the exception of relative productivity calculations that were done exclusively for the plots in the mixing experiment.
Data collection
Estimation of leaf area, the vertical distribution of leaf area, and the vertical distribution of leaf area density
To estimate the individual tree leaf area, we used the Adelaide technique, described by Andrew et al., (1979) and validated by O'Grady et al., (2000). In this field campaign, we used a frame, containing nine horizontal lines, mounted on a tripod to divide an individual tree crown into ten horizontal layers. For each species, we selected 25 trees at each site that covered the range in tree size. On each of these trees, an individual crown unit was determined as a reference. For each tree, we counted the number of units per layer and sampled this reference afterwards to determine the weight, specific leaf area, and hence leaf area of a single unit. For the leaves of F. sylvatica, we used a leaf area metre (LI‑3050C Transparent Belt Conveyor Accessory (LI-COR Biosciences GmbH, Bad Homburg, Germany)) and for P. menziesii we used a flat-bed scanner combined with the WinFOLIA™ Reg 2013a software (Régent Instruments, Quebec City, Canada). In addition, we measured the diameter at breast height (DBH, 1.3 m), height, and live crown length of target trees and their neighbours within a radius of 10 m. Using these data and the specific wood density (WD) of both species, we calculated a competition index (Forrester et al. 2017),
where ba is the cumulative basal area of all individual tree stems at 1.30 m per species within a radius (r) of 10 m and WD is the species-specific wood density (g cm−3). This index was used to develop an allometric equation (Eq. 2) to calculate the crown diameter as a function of the competition index (CI), DBH, and relative height (rh) (height of the target tree divided by the mean height of its neighbours), and we used the mean wood density from values found in the literature and in the Global Wood Density Database (Zanne et al. 2009), for P. menziesii (Alden 1997; CABI, 2017) and F. sylvatica (Barbaroux 2002; Cienciala et al. 2005; Gryc et al. 2008; Skovsgaard and Nord-Larsen 2012)
where Y is crown diameter and ε is a correction ratio used when back-transforming the ln-transformed Y-variables. The correction ratio was calculated as the sum of the measured values divided by the sum of the (back-transformed) predicted values (Snowdon 1991).
The inclusion of a competition index was important because the allometric relationships used to predict some tree data, such as crown diameter in this case, can be influenced by stand structure and composition, as expressed by characteristics such as competition, relative height, and species richness (Forrester et al. 2017). This study also found that interspecific differences in response to competition can be related to the wood density of tree species such that species with higher wood density responded to increased competition by developing larger crowns, whereas species with lower wood density developed smaller crowns (Forrester et al. 2017). Since wood density may moderate the influence of competition, we included it in our calculations.
The leaf area density (LAD, m2 m−3) was calculated for the 10 crown layers. Each layer represented 10% of the live crown length and was assumed to have a volume of a semi-elliptical cone for the top layer or a frustum of a semi-elliptical cone for the other nine layers,
The vertical distribution of LAD was fitted to a beta distribution (Wang et al. 1990),
where a, b, and cc are fitted parameters, and Y is the leaf area density (units) at a relative height of x within the canopy. The LAD of one layer was a function of its relative height within the crown. The parameters of this equation are inputs required to run the MAESTRA model, which was used to calculate individual tree APAR.
Field measurements and further calculations to describe the structure and growth at the tree and stand levels.
We mapped stem positions and measured the DBH of all trees within the triplets, all trees in the density plots, and the next two tree lines around each plot. For 10 trees per species in each plot, we also measured height (h), live crown length (lcl) and crown diameter (cd). These variables were then determined for all other trees by fitting these variables as a function of DBH,
where Y is h, lcl, or cd, and ε is a correction ratio used when back-transforming the ln-transformed Y-variables. The correction ratio was calculated following Snowdon (1991). Individual crown volumes (m3) were calculated for each tree from their crown diameter (cd) and lcl by assuming semi-elliptical shapes (Eq. 3). Separate equations were developed for each species and each site.
The basal area increment of individual trees (cm2 year−1) was calculated using the DBH measurements of the triplet inventory in 2013 (see Thurm et al. 2016) and the density inventory in 2015 (see Nickel and Rais 2015). For the calculation of the individual leaf area (LA) (m2), we used allometric equations according to the general format as in Eq. 6 with corresponding variables (X1, Xn), parameter values (ß0, ß1), and correction ratios (ε) provided in Appendix (Table A1). These were developed in similar stands to this study and also covered the range of our data in terms of DBH, basal area (m2 ha−1), tree density (trees ha−1), and age.
The specific leaf area (SLA in m2 kg−1) was calculated for each species as a function of age using equations (Eq. 7). The respective leaf area results were then averaged. When leaf mass was the response variable, it was previously divided by the SLA,
The parameters used in Eq. 7 for F. sylvatica and P. menziesii are provided in Appendix A (Eq. A1 and Eq. A2). Stand variables calculated included the relative height, mean stand height (m), tree density (trees ha−1), leaf area index (m2 m−2), basal area (m2 ha−1), basal area increment (m2 ha−1 year−1), canopy depth (m), canopy volume (m3 ha−1), and canopy leaf area density (m2 m−3). The relative height of a given species was calculated as the mean height of the target species divided by the mean height of the total stand.
Climate data
All meteorological data were obtained from the ERA-Interim reanalysis daily dataset produced by the European Centre for Medium-Range Weather Forecasts (ECMWF) with a high spatial resolution (0.125° × 0.125) (Dee et al. 2011). The climatic variables included monthly and daily mean temperature, precipitation, solar radiation, and number of frost days. For all plots, we extracted daily data of surface solar radiation downwards (SSRD), from 2012 to 2017 and monthly climate data from 1988 to 2017.
MAESTRA light modelling to estimate APAR
Individual tree APAR was calculated using the MAESTRA model (Grace et al. 1987; Wang and Jarvis 1990; Medlyn 2004; Duursma and Medlyn 2012). This 3D tree-level model calculates the APAR of individual trees based on their crown architecture (e.g. crown dimensions and leaf area and leaf angle distributions), leaf optical properties (LAD and distribution, leaf transmittance, and reflectance), and the shading from neighbouring trees based on the positions of other trees within the plot (defined by x and y coordinates and slope and aspect of the site) and their crown architectures. The growing season was defined by the site-specific phenology of the leaf-bearing period of F. sylvatica. For P. menziesii, APAR was calculated for the growing season (defined by the leaf-bearing period for F. sylvatica) and also for the whole year. The parameterization data are provided in Appendix (Table A3). To validate the APAR predictions, we compared the stand APAR calculated using MAESTRA with that calculated from hemispherical photos that were analysed using the Hemisfer software (Schleppi et al. 2007; Thimonier et al. 2010). The APAR values of both methods were highly correlated (R2 = 0.91) and indicated that MAESTRA underestimated Hemisfer-APAR by only 6.8% (Appendix Fig. A1). This level of accuracy is relatively high, and the slight underestimation by MAESTRA was considered to be acceptable. MAESTRA APAR predictions have also been validated in several other studies of mixtures (Charbonnier et al. 2013; le Maire et al. 2013; Forrester et al. 2018, 2019). The detailed 3-D representation of foliage distributions by MAESTRA enables it to accurately account for the influence of the sun zenith angle on canopy clumping. For example, it has been found to accurately reproduce the direct transmittance zenith profile, gap fraction, between solar zenith angles of 0 to about 70° (Braghiere et al. 2020). Individual tree APAR (GJ) was calculated for the growing season and the entire year. These were averaged (per year) for the entire study period (beginning of 2012 to the end of 2017). The stand APAR for the whole plot or for each species was calculated as the sum of the individual tree APAR of a given plot or the respective species.
Estimation of LUE and relative productivity (RP)
The LUE of individual trees (cm2 GJ−1) was calculated as the basal area increment (cm2 year−1) per unit of APAR (GJ year−1). Stand-level LUE (m2 GJ−1) was calculated as the stand basal area increment (m2 ha−1 year−1) per unit of APAR (GJ ha−1 year−1).
The relative productivity (Eq. 8 and 9) quantifies whether mixing led to an increase (RP > 1) or decrease (RP < 1) in a given variable compared to a monoculture at the stand level per species (Eq. 9) or the whole plot community (Eq. 8) (Williams and McCarthy 2001; Forrester and Pretzsch 2015). The RP calculation also accounts for the proportion of the species in the mixtures, which was quantified in terms of LAI or stand basal area, depending on the response variable. For response variables including tree density, basal area, and basal area increment, we quantified the species proportions in terms of stand basal area. All other proportions for the canopy and light variables (canopy depth, canopy volume, proportion of canopy filled, LAD, APAR, and LUE) were quantified in terms of LAI because it is considered to be more representative of the species’ contributions to light dynamics, and species proportions by leaf area have been shown to provide realistic estimates of species proportions on an area basis (Dirnberger and Sterba 2014).
Equation 8 (\({p}_{{mix}^{sp1, sp2}}\)) calculates the productivity (or the performance of other response variables) of the mixed-species stand in relation to the productivity of their related monocultures (\({p}_{{mono}^{sp1}}\), \({p}_{{mono}^{sp2}}\)) and their proportional (\({\colon\colon }_{{\text{sp}}1}\), \({\colon\colon }_{{\text{sp}}2}\)) amount of LAI or stand basal area. Equation 9 is used to determine the relative productivity by species where (\({p}_{{mix}^{sp1, (sp2)}}\)) is the productivity of a given species (sp1) in a mixture with (sp2).
Results
Tree-level effects of mixing, density, and stand structure
For F. sylvatica, crown variables such as crown diameter, live crown length, and crown volume were larger in mixtures than in monocultures (Table 2). F. sylvatica trees were also significantly taller in the mixed stands than in monocultures even though the taller P. menziesii in the mixtures reduced the relative height of F. sylvatica in the stand (Table 2).
In contrast to F. sylvatica, mixing did not affect the crown parameters for P. menziesii. The growth and APAR of individual P. menziesii were much higher in the mixture than in monoculture, while the growth and APAR of F. sylvatica trees were similar in both treatments (Table 2). As a result, there were no treatment effects on LUE (Table 3).
There were no significant differences between P. menziesii density treatments for any variables (Table 4).
Individual tree APAR was positively correlated with tree leaf area and relative height for both species (Fig. 2). For a given tree leaf area, APAR was greater in mixtures than in monocultures for P. menziesii, but for F. sylvatica APAR remained the same as in monoculture (Fig. 2). There was no influence of LAI on individual tree APAR (Appendix Table A5).
The effect of mixing on the relationship between tree leaf area (a) and relative height (b) for F. sylvatica and P. menziesii and the predicted APAR of individual trees per growing season. Data were fitted to (Appendix Eq. A4), and the statistical information is provided in (Appendix Table A5). While there were no significant effects of mixing (M in Table A5) for F. sylvatica, the lines in the graph could differ slightly because we used the actual means of each variable from the given treatments to plot these lines
Stand-level effects of mixing and density
There were no significant differences between mixtures and monocultures for any stand variables except annual APAR, which was highest in P. menziesii monocultures and lowest in F. sylvatica monocultures (Table 3). There were no significant differences between P. menziesii density treatments for any variables (Table 5).
Relative Productivity at the stand level
The relative mixing effects, in terms of RP, showed that F. sylvatica benefited from the mixture in terms of nearly all variables, except for APAR (Table 3). P. menziesii also benefited in terms of canopy depth, LAD, growth, and LUE, but not in terms of basal area and canopy volume. The results indicate that the respective proportions of the two species make much better use of the light (LUE) in mixed stands compared to their monocultures (RP = 2.91 F.syl | RP = 1.61 P.men) and the LUE of the total stand was slightly higher in mixtures. In addition, there was a small increase in growth (BAI) for each species in the mixture and the total stand (Table 3). For the variables Trees per hectare and Basal area, F. sylvatica also achieved values with RP > 1, while P. menziesii had RP < 1, which can be attributed to the proportions of the two species in the mixture, as there were many smaller F. sylvatica and fewer but larger P. menziesii (Table 3). When APAR was evaluated, F. sylvatica and P. menziesii showed nearly the same relative productivity in mixed stands as in the corresponding monocultures (Table 3).
Discussion
P. menziesii and F. sylvatica made better use of the light in the mixture, as indicated by the relative productivity index (Table 3). The F. sylvatica trees were able to increase their height and crown dimensions below the P. menziesii to the extent that they grew and absorbed light as well as the F. sylvatica trees in the pure stand. The trees of P. menziesii, which were much better exposed in the mixture compared to their pure stand, achieved a significant increase in APAR (85.2%) and basal area growth (185.2%), but this was not sufficient to achieve a higher productivity for the whole mixed stand. Therefore, we could not confirm hypothesis 1: When growth is higher in the mixture, it is associated with increases in both APAR and LUE. Very few other studies have quantified mixing effects on growth and APAR or LUE along site gradients (Forrester and Albrecht 2014; Forrester et al. 2018). One of these studies examined Picea abies and Abies alba mixtures and found that for a given tree size, APAR (for both species) and LUE (of A. alba trees) were greater in mixtures than in monocultures and this effect increased as climatic conditions improved and on sites with faster-growing trees (Forrester and Albrecht 2014). The second study examined Pinus sylvestris and F. sylvatica mixtures and found that mixtures had greater APAR than monocultures, on average, and this effect increased with site productivity. However, this was mainly due to mixing effects on P. sylvestris (Forrester et al. 2018). Mixing or complementarity effects on growth can increase with increasing site quality because light-related interactions are likely to become more important as site quality increases. That is, as soil resources or climatic conditions improve, stand density or stand leaf area may increase, thereby increasing competition for light and the potential benefit of species interactions that improve APAR or LUE. However, in this study, the site quality was similar across all sites, which may explain the small influence of site mixing effects.
Our hypothesis 2 was that intra-specific differences in crown architecture (in addition to interspecific differences) contribute to increases in APAR and LUE in mixtures. Several studies have found that individual tree APAR is strongly related to individual tree LA, relative height and stand LAI and that mixing could modify these relationships (Forrester et al. 2018, 2019). However, there were no mixing effects on APAR for P. menziesii and F. sylvatica in mixtures, nor were there any mixing effects on relationships between individual tree foliage area and APAR, or between individual tree stem diameter and foliage area, or between individual tree stem diameter and crown diameter or crown length.
In mixtures, we observed that for a given tree foliage area, P. menziesii had a greater growing season APAR than F. sylvatica (Fig. 2). F. sylvatica had lower APAR per foliage area because it was the shorter species in the mixture, although F. sylvatica had higher foliage area for given stem diameter. The differences in phenology (deciduous vs. evergreen) did not affect the APAR of P. menziesii, because P. menziesii was taller than the deciduous F. sylvatica and hence its crowns also received high amounts of radiation during the growing season. Similarly, Pinus sylvestris benefited little from the deciduous characteristics of F. sylvatica in mixtures across Europe (Forrester et al. 2018). In contrast, differences in phenology were shown to play a significant role in the mixing effects on APAR in tropical forests where there was less distinct canopy stratification (Sapijanskas et al. 2014).
For both species, relationships between individual tree foliage area and APAR were linear indicating that all trees had a similar APAR relative to their foliage area. A review including data from several hundred plots and at least seven species found a range of linear and exponential relationships, showing that while competition for light is sometimes asymmetric (exponential foliage area – APAR relationships), this is often not the case (linear relationships) (Forrester 2019).
Our hypothesis 3 was that thinning or low stand densities, while increasing individual tree growth owing to increased tree APAR, only influence LUE under site conditions where these treatments also increase the availability of soil resources (e.g. unlikely on sites that are nutrient-rich and moist). However, the P. menziesii spacing experiment showed no significant differences in stand growth, APAR, or LUE between these two sites, and therefore, this hypothesis could not be tested. The lack of planting density effects is probably because the large initial differences in planting density were gradually lost over the 46 years due to thinning, which was more intense in the plots with initially higher planting densities, thereby removing the density effect. Studies on Eucalyptus nitens and Eucalyptus regnans showed that the positive thinning effects on LUE are suppressed when sufficient resources are not available (e.g. nutrients, water) or the competition for resources becomes more intense (West and Osler 1995; Forrester et al. 2012a). Thus, it remains an open question whether thinning or low stand densities can increase stand LUE on sites with a limited supply of soil resources.
This study shows the value of comparing tree- and stand-level growth, APAR and LUE in mixtures, and at different densities, to understand how species interact in mixtures. Mixing can modify APAR and LUE by combining, or modifying, various structural and physiological characteristics, including contrasting phenology (e.g. deciduous vs. evergreen) or differences in crown shape, crown size, crown leaf area density, as well as different tolerances to shade (Ishii and Asano 2010; Niinemets 2010; Sapijanskas et al. 2014; Forrester et al. 2018; Williams et al. 2021; Plaga et al. 2023). These studies also indicate that there is a strong context dependency on the effects. That is, the light-related interactions that have the greatest influence on growth depend on the species that are mixed, as well as age, stand density, soil, and climatic characteristics. This implies a potential for using forest growth models that incorporate and hence summarize the influence of crown and canopy structure on APAR and how this is influenced by site characteristics and management. For this purpose, it will be important that such models have been validated for their ability to predict mixing effects in forests (e.g. Bouwman et al. 2021).
Conclusion
The complementarity of canopy structure and related effects on APAR and LUE in these two species benefits P. menziesii concerning APAR, and most importantly, mixing does not lead to decreased vitality or growth in either of the two species. Since mixing is spreading potential risks from stress or disturbances and may also improve the nutrient use of these two species (Pretzsch 2014; Thurm et al. 2017), these forests might be more resilient, which is becoming an increasingly important attribute. However, this should be tested for other contexts in which this species combination can occur in terms of site conditions, age, stand density or mixing pattern. Since P. menziesii will likely increase in importance in Central European forests, there is a need to also explore other and more diverse species combinations with this species to reduce potential risks of its cultivation.
Data availability
Data are available from the corresponding author on request.
Code availability
Code is available from the corresponding author on request.
References
Alden HA (1997) Softwoods of North America. United States Department of Agriculture, Forest Service, Forest Products Laboratory. Gen. Tech. Report FPL-GTR-102
Ammer C (2019) Diversity and forest productivity in a changing climate. New Phytol 221:50–66. https://doi.org/10.1111/nph.15263
Andrew M, Noble I, Lange R (1979) A non-destructive method for estimating the weight of forage on shrubs. Rangel J 1:225. https://doi.org/10.1071/rj9790225
Barbaroux C (2002) Analyse et modélisation des flux de carbone de peuplements for- estiers pour la compréhension de la croissance de deux espèces feuillues Quercus petraea et Fagus sylvatica. University of Paris-Sud, Orsay
Barbeito I, Dassot M, Bayer D et al (2017) Terrestrial laser scanning reveals differences in crown structure of Fagus sylvatica in mixed vs. pure European forests. For Ecol Manage 405:381–390. https://doi.org/10.1016/j.foreco.2017.09.043
Bauhus J, Van Winden AP, Nicotra AB (2004) Aboveground interactions and productivity in mixed-species plantations of Acacia mearnsii and Eucalyptus globulus. Can J for Res 34:686–694. https://doi.org/10.1139/x03-243
Bauhus J, Forrester DI, Pretzsch H et al (2017) Silvicultural options for mixed-species stands. In: Pretzsch H, Forrester DI, Bauhus J (eds) Mixed-species forests: ecology and management. Springer, Berlin, pp 433–501
Bindewald A, Miocic S, Wedler A, Bauhaus J (2021) Forest inventory—based assessments of the invasion risk of Pseudotsuga menziesii ( Mirb.) Franco and Quercus rubra L. in Germany. Eur J for Res 140:883–899. https://doi.org/10.1007/s10342-021-01373-0
Binkley D, Dunkin KA, DeBell D, Ryan MG (1992) Production and nutrient cycling in mixed plantations of Eucalyptus and Albizia in Hawaii. For Sci 38:393–408. https://doi.org/10.1093/forestscience/38.2.393
Bouwman M, Forrester DI, Ouden Jd, Nabuurs G-J, Mohren GMJ (2021) Species interactions in mixed stands of Pinus sylvestris and Quercus robur in the Netherlands: competitive dominance shifts in favor of P. sylvestris under projected climate change. For Ecol Manage 481:118615
Braghiere RK, Quaife T, Black E et al (2019) Underestimation of global photosynthesis in earth system models due to representation of vegetation structure global biogeochemical cycles. Glob Biogeochem Cycles. https://doi.org/10.1029/2018GB006135
Braghiere RK, Quaife T, Black E, Ryu Y, Chen Q, Kauwe MGD, Baldocchi D (2020) Influence of sun zenith angle on canopy clumping and the resulting impacts on photosynthesis. Agric for Meteorol 291:108065
Bravo F, Fabrika M, Ammer C, Barreiro S, Bielak K, Coll L, Fonseca T, Kangur A, Löf M, Merganičová K, Pach M, Pretzsch H, Stojanović D, Schuler L, Peric S, Rötzer T, Río M, Dodan M, Bravo-Oviedo A (2019) Modelling approaches for mixed forests dynamics prognosis. Research gaps and opportunities. Forest Syst 28: eR002
Brumme R, Khanna PK (2009) Functioning and management of European beech ecosystems. Ecol Stud 208:499. https://doi.org/10.1007/b82392
CABI (2022) Pseudotsuga menziesii (Douglas-fir). CABI Compend CABI Compe. https://doi.org/10.1079/cabicompendium.45266
Charbonnier F, le Maire G, Dreyer E et al (2013) Competition for light in heterogeneous canopies: application of MAESTRA to a coffee (coffea arabica L.) agroforestry system. Agric for Meteorol 181:152–169. https://doi.org/10.1016/j.agrformet.2013.07.010
Cienciala E, Černý M, Apltauer J, Exnerová Z (2005) Biomass functions applicable to European beech. J for Sci 51:147–154. https://doi.org/10.17221/4553-jfs
Dee DP, Uppala SM, Simmons AJ et al (2011) The ERA-Interim reanalysis: configuration and performance of the data assimilation system. Q J R Meteorol Soc 137:553–597. https://doi.org/10.1002/qj.828
Dirnberger GF, Sterba H (2014) A comparison of different methods to estimate species proportions by area in mixed stands. For Syst 23:534–546. https://doi.org/10.5424/fs/2014233-06027
Duursma RA, Medlyn BE (2012) MAESPA: A model to study interactions between water limitation, environmental drivers and vegetation function at tree and stand levels, with an example application to [CO2] × drought interactions. Geosci Model Dev 5:919–940. https://doi.org/10.5194/gmd-5-919-2012
Eilmann B, Rigling A (2012) Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol 32:178–187. https://doi.org/10.1093/treephys/tps004
Forrester DI (2019) Linking forest growth with stand structure: tree size inequality, tree growth or resource partitioning and the asymmetry of competition. For Ecol Manage 447:139–157. https://doi.org/10.1016/j.foreco.2019.05.053
Forrester DI, Albrecht AT (2014) Light absorption and light-use efficiency in mixtures of Abies alba and Picea abies along a productivity gradient. For Ecol Manage 328:94–102. https://doi.org/10.1016/j.foreco.2014.05.026
Forrester DI, Bauhus J (2016) A review of processes behind diversity—productivity relationships in forests. Curr for Reports 2:45–61. https://doi.org/10.1007/s40725-016-0031-2
Forrester DI, Pretzsch H (2015) Tamm Review: on the strength of evidence when comparing ecosystem functions of mixtures with monocultures. For Ecol Manage 356:41–53. https://doi.org/10.1016/j.foreco.2015.08.016
Forrester DI, Bauhus J, Cowie AL (2005) Nutrient cycling in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. Can J for Res 35:2942–2950. https://doi.org/10.1139/x05-214
Forrester DI, Bauhus J, Cowie AL (2006) Carbon allocation in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. For Ecol Manage 233:275–284. https://doi.org/10.1016/j.foreco.2006.05.018
Forrester DI, Theiveyanathan S, Collopy JJ, Marcar NE (2010) Enhanced water use efficiency in a mixed Eucalyptus globulus and Acacia mearnsii plantation. For Ecol Manage 259:1761–1770. https://doi.org/10.1016/j.foreco.2009.07.036
Forrester DI, Collopy JJ, Beadle CL, Baker TG (2012a) Interactive effects of simultaneously applied thinning, pruning and fertiliser application treatments on growth, biomass production and crown architecture in a young Eucalyptus nitens plantation. For Ecol Manage 267:104–116. https://doi.org/10.1016/j.foreco.2011.11.039
Forrester DI, Lancaster K, Collopy JJ et al (2012b) Photosynthetic capacity of Eucalyptus globulus is higher when grown in mixture with Acacia mearnsii. Trees Struct Funct 26:1203–1213. https://doi.org/10.1007/s00468-012-0696-5
Forrester DI, Collopy JJ, Beadle CL, Baker TG (2013) Effect of thinning, pruning and nitrogen fertiliser application on light interception and light-use efficiency in a young Eucalyptus nitens plantation. For Ecol Manage 288:21–30. https://doi.org/10.1016/j.foreco.2011.11.024
Forrester DI, Benneter A, Bouriaud O, Bauhus J (2017) Diversity and competition influence tree allometric relationships – developing functions for mixed-species forests. J Ecol 105(3):761–774. https://doi.org/10.1111/1365-2745.12704
Forrester DI, Ammer C, Annighöfer PJ et al (2018) Effects of crown architecture and stand structure on light absorption in mixed and monospecific Fagus sylvatica and Pinus sylvestris forests along a productivity and climate gradient through Europe. J Ecol 106:746–760. https://doi.org/10.1111/1365-2745.12803
Forrester DI, Rodenfels P, Haase J et al (2019) Tree-species interactions increase light absorption and growth in Chinese subtropical mixed-species plantations. Oecologia 191:421–432. https://doi.org/10.1007/s00442-019-04495-w
Grace JC, Jarvis PG, Norman JM (1987) Modelling the interception of solar radiant energy in intensively managed stands. New Zeal J for Sci 17:193–209
Grossiord C, Granier A, Ratcliffe S et al (2014) Tree diversity does not always improve resistance of forest ecosystems to drought. Proc Natl Acad Sci U S A 111:14812–14815. https://doi.org/10.1073/pnas.1411970111
Gryc V, Vavrčík H, Gomola Š (2008) Selected properties of European beech (Fagus sylvatica L). J for Sci 54:418–425. https://doi.org/10.17221/59/2008-jfs
Ishii H, Asano S (2010) The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests. Ecol Res 25:715–722. https://doi.org/10.1007/s11284-009-0668-4
Jactel H, Bauhus J, Boberg J et al (2017) Tree diversity drives forest stand resistance to natural disturbances. Curr for Rep 3:223–243. https://doi.org/10.1007/s40725-017-0064-1
Kleinschmit J, Bastien J (1992) IUFRO’s role in douglas-fir (Pseudotsuga Menziesii (Mirb.) Franco) tree improvement. Silvae Genet 41:161–173
le Maire G, Nouvellon Y, Christina M et al (2013) Tree and stand light use efficiencies over a full rotation of single- and mixed-species Eucalyptus grandis and Acacia mangium plantations. For Ecol Manage 288:31–42. https://doi.org/10.1016/j.foreco.2012.03.005
Leuschner C, Ellenberg H (2017) Ecology of Central European Forests: Vegetation Ecology of Central Europe
Medlyn B (2004) A MAESTRO retrospective. In: Mencuccini M, Grace J, Moncrieff JB, McNaughton KG (eds) Forests at the land-atmosphere interface. CAB International 2004, Sydney, pp 105–121
Messier C, Bauhus J, Sousa-silva R et al (2022) For the sake of resilience and multifunctionality, let’s diversify planted forests. Conserv Lett. https://doi.org/10.1111/conl.12829
Mezei P, Jakuš R, Pennerstorfer J et al (2017) Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—an infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric for Meteorol 242:85–95. https://doi.org/10.1016/j.agrformet.2017.04.004
Nickel M, Rais A (2013) Exkursionsführer Douglasien-Standraum- und Durchforstungsversuch ANS 609. -Forstbetrieb Rothenburg-. In: Exkursionsführer MWW-EF 77/2, 77/2. p 19
Nickel M, Rais A (2015) Exkursionsführer. Douglasien-Standraum- und Durchfors- tungsversuch HEG 608. -Forstbetrieb Heigenbrücken. In: Exkursionsführer MWW-EF 95/3, 95/3. p 15
Niinemets (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res 25:693–714. https://doi.org/10.1007/s11284-010-0712-4
O’Grady AP, Chen X, Eamus D, Hutley LB (2000) Composition, leaf area index and standing biomass of eucalypt open forests near Darwin in the Northern Territory, Australia. Aust J Bot 48:629–638. https://doi.org/10.1071/BT99022
Plaga BNE, Bauhus J, Smith AR, Pereira MG, Forrester DI (2023) Drought-related mortality modifies mixing effects on light absorption and growth in mono-specific and mixed stands of Fagus sylvatica, Alnus glutinosa and Betula pendula. Forestry doi:https://doi.org/10.1093/forestry/cpad051
Pretzsch H (2014) Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For Ecol Manage 327:251–264. https://doi.org/10.1016/j.foreco.2014.04.027
Pretzsch H, Forrester DI, Rötzer T (2015) Representation of species mixing in forest growth models. A review and perspective. Ecol Model 313:276–292
Sapijanskas J, Paquette A, Potvin C et al (2014) Tropical tree diversity enhances light capture through crown plasticity and spatial and temporal niche differences. Ecology 95:2479–2492. https://doi.org/10.1890/13-1366.1
Schleppi P, Conedera M, Sedivy I, Thimonier A (2007) Correcting non-linearity and slope effects in the estimation of the leaf area index of forests from hemispherical photographs. Agric for Meteorol 144:236–242. https://doi.org/10.1016/j.agrformet.2007.02.004
Skovsgaard JP, Nord-Larsen T (2012) Biomass, basic density and biomass expansion factor functions for European beech (Fagus sylvatica L.) in Denmark. Eur J for Res 131:1035–1053. https://doi.org/10.1007/s10342-011-0575-4
Snowdon P (1991) A ration estimator for bias correction in logarithmic regressions. Can J for Res 21:720–724. https://doi.org/10.1139/x91-101
Thimonier A, Sedivy I, Schleppi P (2010) Estimating leaf area index in different types of mature forest stands in Switzerland: a comparison of methods. Eur J for Res 129:543–562. https://doi.org/10.1007/s10342-009-0353-8
Thurm EA, Pretzsch H (2016) Improved productivity and modified tree morphology of mixed versus pure stands of European beech (Fagus sylvatica) and Douglas-fir (Pseudotsuga menziesii) with increasing precipitation and age. Ann for Sci 73:1047–1061. https://doi.org/10.1007/s13595-016-0588-8
Thurm EA, Uhl E, Pretzsch H (2016) Mixture reduces climate sensitivity of Douglas-fir stem growth. For Ecol Manage 376:205–220. https://doi.org/10.1016/j.foreco.2016.06.020
Thurm EA, Biber P, Pretzsch H (2017) Stem growth is favored at expenses of root growth in mixed stands and humid conditions for Douglas-fir (Pseudotsuga menziesii) and European beech (Fagus sylvatica). Trees Struct Funct 31:349–365. https://doi.org/10.1007/s00468-016-1512-4
Vandermeer JH (1989) The ecology of intercropping, 1. publ. Cambridge University Press, New York
Vitali V, Büntgen U, Bauhus J (2017) Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob Chang Biol 23:5108–5119. https://doi.org/10.1111/gcb.13774
Wang YP, Jarvis PG (1990) Description and validation of an array model - MAESTRO. Agric for Meteorol 51:257–280. https://doi.org/10.1016/0168-1923(90)90112-J
Wang YP, Jarvis PG, Benson ML (1990) Two-dimensional needle-area density distribution within the crowns of Pinus radiata. For Ecol Manage 32:217–237. https://doi.org/10.1016/0378-1127(90)90172-8
West PW, Osler GHR (1995) Growth response to thinning and its relation to site resources in Eucalyptus regnans. Can J for Res 25:69–80. https://doi.org/10.1139/x95-009
Williams AC, McCarthy BC (2001) A new index of interspecific competition for replacement and additive designs. Ecol Res 16:29–40
Williams LJ, Butler EE, Cavender-Bares J, Stefanski A, Rice KE, Messier C, Paquette A, Reich PB (2021) Enhanced light interception and light use efficiency explain overyielding in young tree communities. Ecol Lett 24:996–1006
Zanne AE, Lopez-Gonzalez G, Coomes DA, et al (2009) Global Wood Density Database - Data from: Towards a worldwide wood economics spectrum
Zhang Y, Chen HYH, Reich PB (2012) Forest productivity increases with evenness, species richness and trait variation: a global meta-analysis. J Ecol 100:742–749. https://doi.org/10.1111/j.1365-2745.2011.01944.x
Acknowledgements
This article is a part of the PhD research of the first author who was funded by the DFG grant (FO 791/5- 1) to D. I. Forrester in the context of his Heisenberg Fellowship (FO 791/4- 1) provided by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG). All contributors thank their national funding institutions and the forest owners for agreeing to run the field campaign in the Bavarian triplet plots and to measure and analyse data from these plots. The contribution of M. G. Pereira to the study was supported by National Funds through FCT—Foundation for Science and Technology under the project UIDB/04033/2020. We are also very grateful to Marie Roggenhofer for helping us during the field campaign and laboratory work.
Funding
Open access funding provided by CSIRO Library Services. Open access funding is enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There is no conflict of interests by any of the authors.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors agreed to publish the results described in this manuscript.
Additional information
Communicated by Ruediger Grote.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plaga, B.N.E., Bauhus, J., Pretzsch, H. et al. Influence of crown and canopy structure on light absorption, light use efficiency, and growth in mixed and pure Pseudotsuga menziesii and Fagus sylvatica forests. Eur J Forest Res 143, 479–491 (2024). https://doi.org/10.1007/s10342-023-01638-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01638-w