Abstract
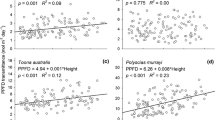
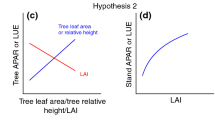
Mixed forests comprising multiple tree species with contrasting crown architectures, leaf phenologies, and photosynthetic activity, tend to have high ecosystem productivity. We propose that in such forests, differentiation among coexisting species in their spatial and temporal strategies for light interception, results in complementary use of light. Spatial differentiation among coexisting tree species occurs as a result of adaptation of crown architecture and shoot/leaf morphology to the spatially variable light conditions of the canopy, sub-canopy, and understory. Temporal differentiation occurs as a result of variation in leaf phenology and photosynthetic activity. The arrangement of leaves in both space and time is an important aspect of plant strategies for light interception and determines photosynthetic carbon gain of the plant canopy. For example, at the shoot level, morphological and phenological differentiation between long and short shoots reflects their respective shoot functions, indicating that spatial and temporal strategies for light interception are linked. Complementary use of light is a consequence of the spatiotemporal differentiation in light interception among coexisting species. Because coexisting species may show differentiation in strategies for resource acquisition (functional diversification) or convergence with respect to some limiting resource (functional convergence), the relative importance of various crown functions and their contribution to growth and survival of individuals need to be evaluated quantitatively and compared among coexisting species.


Similar content being viewed by others
References
Aiba S, Hanya G, Tsujino R, Takyu M, Seino T, Kimura K, Kitayama K (2007) Comparative study of additive basal area of conifers in forest ecosystems along elevational gradients. Ecol Res 22:439–450
Asano S (2007) Phenology of current-year shoots of Rhododendron reticulatum and R. macrosepalum in a secondary forest in southern Kyoto Prefecture. Graduate School of Agricultural Science. Kobe University, Kobe
Aubin I, Beaudet M, Messier C (2000) Light extinction coefficients specific to the understory vegetation of the southern boreal forest, Quebec. Can J For Res 30:168–177
Augspurger CK (2008) Early spring leaf out enhances growth and survival of saplings in a temperate deciduous forest. Oecologia 156:281–286
Augspurger CK, Bartlett EA (2003) Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol 23:517–525
Caesar JC, MacDonald AD (1984) Shoot development in Betula papyrifera. IV. Comparisons between growth characteristics and expression of vegetative long and short shoots. Can J Bot 62:446–453
Cai ZQ, Schnitzer SA, Bongers F (2009) Seasonal differences in leaf-level physiology give lianas a competitive advantage over trees in a tropical seasonal forest. Oecologia 161:25–33
Canham CD (1988) Growth and canopy architecture of shade-tolerant trees: response to canopy gaps. Ecology 69:786–795
Cannell MGR, Malcolm DC, Robertson PA (1992) The ecology of mixed-species stands of trees. Blackwell Scientific Publications, Oxford
Chen JM, Rich PM, Gower ST, Norman JM, Plummer S (1997) Leaf area index of boreal forests: theory, techniques, and measurements. J Geophys Res 102:29429–29443
Enright NJ, Ogden J, Rigg LS (1999) Dynamics of forests with Araucariaceae in the western Pacific. J Veg Sci 10:793–804
Farnsworth KD, Niklas KJ (1995) Theories of optimization, form and function in branching architecture in plants. Funct Ecol 9:355–363
Franklin JF, Maeda T, Ohsumi Y, Matsui M, Yagi H (1979) Subalpine coniferous forests of central Honshu, Japan. Ecol Monogr 49:311–334
Goto Y, Kominami Y, Miyama T, Tamai K, Kanazawa Y (2003) Aboveground biomass and net primary production of a broad-leaved secondary forest in the southern part of Kyoto Prefecture, central Japan. Bull For For Prod Res Inst 2:115–147
Harada Y, Takada T (1988) Optimal timing of leaf expansion and shedding in a seasonally varying environment. Plant Species Biol 3:89–97
Hattori T, Nakanishi S (1985) On the distribution limits of lucidophyllous forest in the Japanese Archipelago. Bot Mag Tokyo 98:317–333
Hiura T, Fujiwara K (1999) Density-dependence and co-existence of conifer and broad-leaved trees in a Japanese northern mixed forest. J Veg Sci 10:843–850
Ishibashi S (2000) The relationship between the distribution of tree species and land description in natural cool-temperate and boreal forests. J Jpn For Soc 82:243–250
Ishii H, Ford ED (2002) Persistence of Pseudotsuga menziesii (Douglas-fir) in temperate coniferous forests of the Pacific Northwest Coast, USA. Folia Geobot 37:63–69
Ishii H, Tanabe S, Hiura T (2004) Exploring the relationships among canopy structure, stand productivity and biodiversity of temperate forest ecosystems. For Sci 50:342–355
Ishii H, Yoshimura K, Mori A (2009) Convergence of leaf display and photosynthetic characteristics of understory Abies amabilis and Tsuga heterophylla in an old-growth forest in southwestern Washington State, USA. Tree Physiol 29:989–998
Ishikawa Y, Ito K (1989) The regeneration process in a mixed forest in central Hokkaido, Japan. Vegetatio 79:75–84
Iwasaki A, Ishii HT (2005) Vegetation structure of fragmented shrine/temple forests in southeastern Hyogo Prefecture––estimation of edge-effect distance and minimum conservation area. Human Nat 15:29–42
Jarvis PG, Leverenz JW (1983) Productivity of temperate, deciduous and evergreen forests. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Ecosystem processes: mineral cycling, productivity and man’s influence. Encyclopedia of plant physiology. Springer, Berlin Heidelberg New York, pp 233–280
Jones M, Harper JL (1987) The influence of neighbors on the growth of trees. II. The fate of buds on long and short shoots in Betula pendula. Proc R Soc Lond Ser B 232:19–33
Kawamura K (2009) A conceptual framework for the study of modular responses to local environmental heterogeneity within the plant crown and a review of related concepts and ideas. Ecol Res (in press)
Kennedy MC (2009) Functional-structural models optimize the placement of foliage units for multiple whole-canopy functions. Ecol Res (in press)
Kikuzawa K (1984) Leaf survival of woody plants in deciduous broad-leaved forests. 2. Small trees and shrubs. Can J Bot 62:2551–2556
Kikuzawa K (1995) Leaf phenology as an optimal strategy for carbon gain in plants. Can J Bot 73:158–163
Kikuzawa K, Ohto Y, Umeki K, Lechowicz MJ (2009) Canopy ergodicity: can a single leaf represent an entire plant canopy? Plant Ecol 202:309–323
Kubota Y, Hara T (1995) Tree competition and species coexistence in a sub-boreal forest, northern Japan. Ann Bot 76:503–512
Leverenz JW, Hinckley TM (1990) Shoot structure, leaf area index, and productivity of evergreen conifer stands. Tree Physiol 6:135–149
Lusk CH, Smith B (1998) Life history differences and tree species coexistence in an old-growth New Zealand rain forest. Ecology 79:795–806
Maeno H, Hiura T (2000) The effect of leaf phenology of overstory trees on the reproductive success of an understory shrub, Staphylea bumalda DC. Can J Bot 78:781–785
Meinzer FC (2003) Functional convergence in plant responses to the environment. Oecologia 134:1–11
Messier C, Parent S, Bergeron Y (1998) Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J Veg Sci 9:511–520
Midgley JJ, Parker R, Laurie H, Seydach A (2002) Competition among canopy trees in indigenous forests: an analysis of the ‘additive basal area’ phenomenon. Aust Ecol 27:269–272
Miyazawa Y, Kikuzawa K (2004) Phenology and photosynthetic traits of short shoots and long shoots in Betula grossa. Tree Physiol 24:631–637
Miyazawa Y, Kikuzawa K (2005) Winter photosynthesis by saplings of evergreen broadleaved trees in a deciduous temperate forest. New Phytol 165:857–866
Monsi M, Saeki T (2005) On the factor light in plant communities and its importance for matter production. Ann Bot 95:549–567
Montserrat-Marti G, Camarero J, Palacio S, Perez-Rontome C, Milla R, Albuixech J, Maestro M (2009) Summer-drought constrains the phenology and growth of two coexisting Mediterranean oaks with contrasting leaf habit: implications for their persistence and reproduction. Trees Struct Funct 23:787–799
Mori A, Takeda H (2004a) Effects of mixedwood canopies on conifer advance regeneration in a subalpine old-growth forest in central Japan. EcoScience 11:36–44
Mori A, Takeda H (2004b) Effects of undisturbed canopy structure on population structure and species coexistence in an old-growth subalpine forest in central Japan. For Ecol Manag 200:89–100
Mori AS, Mizumachi E, Komiyama A (2007) Roles of disturbance and demographic non-equilibrium in species coexistence, inferred from 25-year dynamics of a late-successional old-growth subalpine forest. For Ecol Manag 241:74–83
Niinemets U (1998) Growth of young trees of Acer platanoides and Quercus robur along a gap-understory continuum: interrelationships between allometry, biomass partitioning, nitrogen, and shade tolerance. Int J Plant Sci 159:318–330
Niinemets U (2009) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res (In press)
Nitta I, Ohsawa M (1997) Leaf dynamics and shoot phenology of eleven warm-temperate evergreen broad-leaved trees near their northern limit in central Japan. Plant Ecol 130:71–88
Norman JM, Jarvis PG (1975) Photosynthesis in sitka spruce (Picea sitchensis (Bong.) Carr.): V. radiation penetration theory and a test case. J Appl Ecol 12:839–878
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Palmroth S, Palva L, Stenberg P, Kotisaari A (1999) Fine scale measurement and simulation of penumbral radiation formed by a pine shoot. Agric For Meteorol 95:15–25
Parker GG (1997) Canopy structure and light environment of an old-growth Douglas-fir/western hemlock forest. Northwest Sci 71:261–270
Parker GG, Harmon ME, Lefsky MA, Chen J, Van Pelt R, Weiss SB, Thomas SC, Winner WE, Shaw DC, Franklin JF (2004) Three-dimensional structure of an old-growth Pseudotsuga-Tsuga canopy and its implications for radiation balance, microclimate, and gas exchange. Ecosystems 7:440–453
Perry DA (1994) Forest ecosystems. Johns Hopkins University Press, Baltimore
Reich PB, Falster DS, Ellsworth DS, Wright IJ, Westoby M, Oleksyn J, Lee TD (2009) Controls on declining carbon balance with leaf age among 10 woody species in Australian woodland: do leaves have zero daily net carbon balances when they die? New Phytol 183:153–166
Seino T (2009) Shoot growth and tree architecture of subcanopy and intermediate-successional status trees. Ecol Res (in press)
Seiwa K (1999) Changes in leaf phenology are dependent on tree height in Acer mono, a deciduous broad-leaved tree. Ann Bot Ann Bot 83:355–361
Sipe TW, Bazzaz FA (1994) Gap partitioning among maples (Acer) in central New England: shoot architecture and photosynthesis. Ecology 75:2318–2332
Smolander S, Stenberg P (2001) A method for estimating light interception by a conifer shoot. Tree Physiol 21:797–803
Smolander S, Stenberg P (2003) A method to account for shoot scale clumping in coniferous canopy reflectance models. Remote Sens Environ 88:363–373
Stenberg P (1998) Implications of shoot structure on the rate of photosynthesis at different levels in a coniferous canopy using a model incorporating grouping and penumbra. Funct Ecol 12:82–91
Sterck FJ (1999) Crown development in tropical rain forest trees in gaps and understory. Plant Ecol 143:89–98
Suzuki E, Tsukahara J (1987) Age structure and regeneration of old growth Cryptomeria japonica forests on Yakushima Island. Bot Mag Tokyo 100:223–241
Takahashi K, Mitsuishi D, Uemura S, Suzuki J-I, Hara T (2003) Stand structure and dynamics during a 16-year period in a sub-boreal conifer-hardwood mixed forest, northern Japan. For Ecol Manag 174:39–50
Takashima A, Masuki J, Mitsuda Y, Yoshida S, Murakami T, Imada M (2003) The structure and dynamics of three permanent plots of natural Cryptomeria japonica stands on Yakushima Island. Kyushu J For Res 56:42–47
Takenaka A (1997) Structural variation in current-year shoots of broad-leaved evergreen tree saplings under forest canopies in warm temperate Japan. Tree Physiol 17:205–210
Takeuchi K, Washitani I, Tsunekawa A (eds) (2003) Satoyama: the traditional rural landscape of Japan. Springer, Berlin Heidelberg New York
Tang C, Ohsawa M (2002) Coexistence mechanisms of evergreen, deciduous and coniferous trees in a mid-montane mixed forest on Mt. Emei, Sichuan, China. Plant Ecol 161:215–230
Therezien M, Palmroth S, Brady R, Oren R (2007) Estimation of light interception properties of conifer shoots by an improved photographic method and a 3D model of shoot structure. Trees 27:1375–1387
Uemura S (1994) Patterns of leaf phenology in forest understory. Can J Bot 72:409–414
Valladares F (2003) Light heterogeneity and plants: from ecophysiology to species coexistence and biodiversity. In: Esser K, Luttge L, Beyschlag W, Hellwig F (eds) Progress in botany. Springer, Berlin Heidelberg New York, pp 439–471
Valladares F, Niinemets U (2007) The architecture of plant crowns: form design rules to light capture and performance. In: Pugnaire F, Valladares F (eds) Functional plant ecology. Taylor & Francis, New York, pp 101–149
Valladares F, Niinemets U (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Syst 39:237–257
Valldares F, Skillman JB, Pearcy RW (2002) Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. Am J Bot 89:1275–1284
VanPelt R, Franklin JF (1999) Response of understory trees to experimental gaps in old-growth Douglas-fir forests. Ecol Appl 9:504–512
Veres JS, Pickett STA (1982) Branching patterns of Lindera benzoin beneath gaps and closed canopies. New Phytol 91:767–772
Warren C (2006) Why does photosynthesis decrease with needle age in Pinus pinaster? Trees Struct Funct 20:157–164
Wilson BF (1991) Shoot-length frequencies in black birch (Betula lenta). Can J For Res 21:1475–1480
Yagi T (2000) Morphology and biomass allocation of current-year shoots of ten tall tree species in cool temperate Japan. J Plant Res 113:171–183
Yagi T (2004) Within-tree variations in shoot differentiation patterns of 10 tall tree species in a Japanese cool-temperate forest. Can J Bot 82:228–243
Yagi T, Kikuzawa K (1999) Patterns in size-related variations in current-year shoot structure in eight deciduous tree species. J Plant Res 112:343–352
Yokozawa M, Kubota Y, Hara T (1996) Crown architecture and species coexistence in plant communities. Ann Bot 78:437–447
Youngblood A (1995) Development patterns in young conifer-hardwood forests of interior Alaska. J Veg Sci 6:229–236
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ishii, H., Asano, S. The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests. Ecol Res 25, 715–722 (2010). https://doi.org/10.1007/s11284-009-0668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-009-0668-4