Abstract
We analyzed growth trajectories recorded in the tree-ring series of Norway spruce Picea abies (L.) H. Karst. and silver fir Abies alba Mill. co-occuring with European beech Fagus sylvatica L. in old-growth forests driven by natural stand dynamics. The study sites were localized in five old-growth stands in the Western Carpathians (Central Europe). We characterized the life histories of 218 individual trees by using 25 metrics which refer to the age of the trees, number of release events, reaction to releases, radial increment and basal area increment. We found a large variation in the dbh-age relationship. The life histories of most trees included two or three (in single cases up to seven) release events. We did not find tree age as an important factor in determining post-release growth response. The maximal basal area increment was frequently registered at the terminal stage of tree life. The life histories of spruces and firs did not significantly differ. We concluded that in natural forests driven by gap-phase dynamics, the history of release events is the primary driver of tree growth and that tree age plays a secondary role. After reaching the overstory, trees can continue growing without significant symptoms of aging until extrinsic forces cause their death. Our results indicate that in the studied ecosystem the persistence of less shade-tolerant spruce is not attributable to the niche differentiation and site partitioning mechanism. An alternative hypothesis based on shifts in stand densities, species composition and climatic factors is more probable.
Similar content being viewed by others
Introduction
The exploitation of forest resources by humans has altered the structure, species composition and dynamics of forests across vast areas of the globe. Forest management frequently reduces the structural complexity of forest ecosystems and fundamentally changes the patterns of forest dynamics (Seymour et al. 2002; Kaufmann et al. 2018; Stiers et al. 2018). As a consequence, the ontogenic development and growth dynamics of trees in managed stands usually differ considerably from those in forests driven by natural stand dynamics (Korpel 1995; Lorimer et al. 2001). This divergence has consequences for the life processes and functioning of trees and their populations, and the primary stress factors they are exposed to (Gray et al. 2012; Morimoto et al. 2019). The ontogeny of trees influences their morphological traits (Schröter et al. 2012; Di Filippo et al. 2017), growth potential (Sillet et al. 2015; Pretzsch 2020), reaction to stress conditions (Domec et al. 2015; Pretzsch 2022; Schmied et al. 2022) and longevity (Woods 2008; Di Filippo et al. 2015; Piovesan et al. 2019). It affects availability of key microhabitats which develop on trees and hence the biodiversity of organisms associated with trees (Moning and Müller 2009; Lindenmayer 2017).
In lower altitudes of montane regions in Europe, forests formed by late-seral, shade-tolerant species European beech Fagus sylvatica L., silver fir Abies alba Mill., and Picea abies (L.) H. Karst dominate (Leuschner and Ellenberg 2017). In managed forests representing this community, the most commonly applied sylvicultural systems are different forms of the shelterwood system with a 15–50-year-long regeneration period, and in some regions also the single-tree or group selection system (Schütz et al. 2016). In the shelterwood system the overstory is regularly reduced by regular, irregular or gap cuttings (Raymond et al. 2009). Harvesting is typically intensified in stand patches with ample regeneration to avoid its long suppression and damage in final stages. This pattern of stand dynamics shapes tree life histories, which are typically characterized by early release from competition from the overstory stand, fast growth, the early culmination of diameter and height increment, short lifetimes (80–140 years) and small terminal dimensions (diameter at breast height of less than 60 cm). The commonly used models of tree growth anticipate the culmination of volume (or mass) increment in middle-aged stands and its subsequent rapid reduction, symptomatic for senile processes (Assmann 1961; Wenk et al. 1990). As a consequence, tree growth runs through clearly distinguishable phases of juvenile growth, optimum and aging.
Natural beech-fir-spruce forests are driven by gap-phase dynamics with occasional more severe disturbances (Splechtna et al. 2005; Nagel et al. 2014; Frankovič et al. 2021; Jastrzębski and Paluch 2022). This type of forest dynamics favors the formation of multi-aged and multi-storied stand structures with a quasi-continuous pattern of generation replacement (Král et al. 2014; Parobeková et al. 2018; Paluch et al. 2015). It may be expected that in such stands the life histories of single trees are much more complex than those in managed forests (Piovesan et al. 2019). A principal reason for this is the potentially long lifetime which, unlike that of trees in managed forests, is not interrupted by logging at an age optimized for economic timber utility. Pretzsch (2020) showed a long continuation of intensive tree growth until the age of several hundred years, which ranges beyond the commonly applied rotation period in managed stands. In managed multi-aged forests stand densities are systematically regulated and typically maintained at a moderate level, whereas in forests driven by natural stand dynamics extreme densities may occur more frequently and both open and dense stand patches may co-occur in the forest landscape (Král et al. 2014; Paluch et al. 2021). As a consequence, the growth patterns and life histories of single trees may considerably differ. The complexity of growth trajectories stems from the occurrence of periods of accelerated and suppressed growth induced by the stochasticity of the gap formation process. Past periods of growth suppression and disturbances may be relevant for the future growth of a tree (Camarero et al. 2018; Pretzsch 2022). Reduced growth rates, starting from the earliest ontogenic stages, can be viewed as a necessary condition for attaining longevity (Bigler and Veblen 2009; Piovesan and Biondi 2021). Tree-ring, crown and root morphology represent a structural memory (Backhaus et al. 2014; Ogle et al. 2015) which may affect light interception, hydraulic conduction or water and nutrient uptake and influence functioning and growth patterns.
Studies conducted on the life histories trees frequently focused on regeneration (Šafar 1954; Jaworski 1979; Wright et al. 2000; Lin et al. 2012), the growth reaction of young trees to disturbances (Stan and Daniels 2010; Stan and Daniels 2014; Vašičková et al. 2016) or intended treatments in the overstory (Pedersen and Howard 2004; Puettmann et al. 2009; Hynynen et al. 2019). The obtained results suggest that age or long periods of suppression do not affect the ability of shade-tolerant species to respond to release. Less shade-tolerant species show varying degrees of response, with the strongest reactions in the least shade-tolerant species (Wright et al. 2000). Most studies reported a lag in response to release, which reflect physiological and morphological adjustments of suppressed trees following overstory removal (Kneeshaw et al. 2002; Claveau et al. 2002; Renninger et al. 2007). Roberts and Harrington (2008) showed that after release young Douglas firs and western hemlocks grew even faster than would have been expected based on residual stand density. Typically, small trees respond with higher relative growth rates compared to larger trees (Metslaid et al. 2007; Baral et al. 2016) because dominant trees are less influenced by neighborhood conditions (Schütz 1975; Puettmann et al. 2009). Several studies have shown that also mature trees can effectively respond to reductions in stand density (e.g., Latham and Tappeiner 2002; Bebber et al. 2004; Puettmann et al. 2009). Nonetheless, knowledge on the life histories of old trees growing in close-to-pristine forests is frequently scarce. This knowledge seems however crucially important for our understanding of the past and future dynamics of natural forest ecosystems. The considerable structural complexity of natural forests, which is often already visible at the scale of neighboring trees, suggests that release events may play a more important role for their structural development than the regeneration processes itself (Senécal et al. 2018; Paluch 2021). They allegedly maintain the persistence of a regeneration bank, but under gap-phase dynamics driven by small treefalls, additional release events are usually needed to cause successful recruitment to the upper stand layer.
In mixed-species stands the scrutiny of life histories of individuals representing different species facilitates the understanding of the ecological mechanisms of species co-existence (Abrams and Orwig 1996; Emborg 2007; Amos-Binks and MacLean 2016). Under a disturbance regime dominated by treefall gaps, the coexistence of tree species appears to be associated with differences in growth rate, shade tolerance, canopy residence time and facility for reproduction (Grassi et al. 2004; Rozenbergar et al. 2007; Firm et al. 2009; Gravel et al. 2010; Nagel et al. 2014). In this study, we analyzed development trajectories recorded in the tree-ring series of two conifer species, Norway spruce and silver fir, which grow with European beech in mixed-species forests driven by gap-phase dynamics. Compared to fir, spruce is better adapted for the continental climate (Leuschner and Ellenberg 2017), is less shade-tolerant but grows faster under conditions of full light availability (Grassi and Bagnaresi 2001; Brunner and Huss 1994), and due to its smaller seeds is a better seed-disperser (Kohlermann 1950). These features suggest that in natural mixed-species forests formed by more shade-tolerant fir and beech, its persistence is associated with the occurrence of more severe disturbances which create larger gaps with better light accessibility. The rapid colonization and fast juvenile growth associated with these events seems be decisive for the final success in competition with shade-tolerant fir and beech. Nonetheless, despite the theoretical plausibility of a site partitioning mechanism, empirical evidence documenting differences in the growth strategies of spruce and fir in conditions of natural stand dynamics is lacking.
In Europe, where well-preserved natural forests remain on very small areas (Sabatini et al. 2018) and are subjected to a strict regime of protection which excludes invasive methods of data acquisition, information on the life histories of individual trees growing in such conditions is particularly rare. To diminish this knowledge gap we analyzed tree-ring series collected mostly from deceased Norway spruce and silver fir trees growing in mixed-species old-growth forests in the Western Carpathians (Central Europe). The main objective of this study was to gain a deeper insight into the development trajectories of trees growing in natural forests driven by gap-phase stand dynamics. We expected that the growth of such trees strongly depends on small-scale disturbances which periodically decrease competition pressure and cause release effects. We hypothesized that (1) the tree-ring chronologies of such trees include sequences of enhanced and slowed growth and that the relationship between tree age and tree size is very weak. We assumed also that (2) the growth of trees, here measured by basal area increment, strongly intensifies after entrance into the upper canopy and culminates at an advanced stage of their life, far beyond the usual rotation age used in managed forest. Because our study included moderate shade-tolerant Norway spruce and shade-tolerant silver fir, we expected that (3) the life trajectories of spruces and firs presently growing in the upper stand layer differ. Specifically, we hypothesized that (3a) Norway spruce exposed to competition with more shade-tolerant species fir and beech might survive due to a site partitioning mechanism in less shaded environments associated with larger canopy openings and more intense disturbances. Therefore, in the life histories of spruce trees, we expected to find evidence of an early release, more rapid growth at a young age and lower canopy access time compared to fir. We also hypothesized that (3b) silver fir as a typical shade-tolerant species is characterized by greater growth plasticity and the ability to persist in shadier environments compared to Norway spruce. We expected to find the manifestation of these differing properties in the more complex life trajectories of firs compared to spruces, and in particular, in the higher frequency of release events, greater variation of tree-ring widths and weaker relationship between size and age.
Methods
Study sites
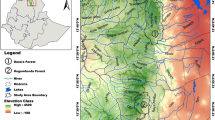
The study was conducted in five old-growth forests in the Polish part of the Western Carpathians (Central Europe) (Fig. 1). Three stands are located in strict reserves (Łabowiec, Oszast, Uhryń) and two are located in national parks (Dolina Łopusznej—hereafter Łopuszna—in Gorczanski NP and Żarnówka in Babia Gora NP). The selected stands are the best preserved mixed-species old-growth forests in the region and are formed by European beech, silver fir and—except for Łabowiec and Uhryń—Norway spruce (Table 1). The lack of Norway spruce in the two latter stands is likely attributable to the generally lower altitude of the mountain ranges and lower precipitation at these locations. All the stands are growing on Cambisols formed from Carpathian flysh. Depending on the altitude and exposition, mean annual temperature ranges between 5.0 and 6.0 °C. Annual precipitation is 900–1450 mm, tends to increase with altitude and from east to west, and reaches a maximum in the summer months (Paszyński and Niedźwiedź 1999; Wypych et al. 2018). The common disturbing factors include windstorms, thunderstorms, ice storms and heavy snowfall in the autumn and spring. Spruce is also frequently attacked and killed by European spruce bark beetle (Ips typographus L.).
The stand fragments selected for this study are free from the stumps of harvested trees, logging trails, plantings or any sign of distortion in forest structure linked with management activity. Nonetheless, we cannot rule out plunder-like cuttings in the past. Although the history of settlement here dates back to the sixteenth century and clear felling was initiated in the region in the middle of the nineteenth century, the studied stands have not experienced such anthropogenic disturbances. The reconstruction of the disturbance regime since 1850 indicates that all the stand fragments where this present research was conducted have been driven by gap-phase dynamics (Jastrzębski and Paluch 2022). Specifically, the disturbance severity approximated by the frequencies of release events recorded in the increment chronologies of single trees varied between 5 and 30% per decade, and the distribution of release signals was slightly over-dispersed in comparison to a Poisson (random) distribution. At present, the forest structure is very heterogeneous (Paluch et al. 2015, 2021) and characterized by a matrix of multilayered structure with scattered canopy openings of variable size and irregular shapes. The mean canopy openness determined for trees of diameter at breast height (dbh) ≥ 25 cm varies between 20 and 41% (Paluch and Jastrzębski 2022). A random component dominates in the spatial pattern of canopy heterogeneity and only 9–39% of the total variation is attributable to structural variation. The trees reach a dbh above 100 cm, heights above 45 m and an age (determined by coring at a height of 130 cm) of above 300 years (Jastrzębski and Paluch 2022). Tree species forming the studied stands are randomly intermingled and do not form clearly distinguishable aggregations of more than several trees. Although, with the exception of the Oszast reserve, beech accounts for the highest proportion in the contemporary species composition (Table 1), historical records indicate that the proportion of conifer species tended to decrease in the studied stands during the twentieth century (Zapałowicz 1880; Jaworski 2016; Paluch et al. 2022).
Field work
Because of ethical issues and regulations which prohibit or restrict the use of invasive research techniques such as tree coring in strict reserves, we attempted to core recently deceased trees with wood in the initial stage of decomposition. In each of the reserves, recently deceased trees representing the upper stand layer (hereafter, upper layer trees) with a dbh equal to or greater than 40 cm and at least 10 m apart were identified. In the studied stands, a dbh of 40 cm approximately corresponds to a height of 27–29 m for fir and spruce, and such trees may be regarded as representing the upper stand layer (> 2/3 of the maximal height) since only single trees reach heights above 42 m. The trunk wood of the chosen dead trees was in the initial stage of decomposition to enable core extraction using an increment drill. Their death was caused by biotic factors (fungi and insects), wind or snow. When the distance between the nearest neighboring dead trees was greater than 30 m, living firs or spruces (in Łabowiec, Oszast and Uhryń) were designated for sampling. Due to formal restrictions, the coring of live trees was unfeasible in the Gorce and Żarnówka reserves in the national parks. In the Łabowiec, Oszast and Uhryń stands, a sample of live trees representing the middle stand layer with dbh between 20 and 39 cm (hereafter, middle layer trees) was also preselected. To equally localize the sampling sites, the middle layer trees were selected every 50 m close to the transect axes. In this case only live trees were considered for sampling because dead trees, which often die due to competitive pressure from larger individuals, most probably would be unrepresentative for the entire population of middle layer exemplars. Depending on the area of the core zone of the reserves and the homogeneity of site conditions, the total area covered by the survey was 7.5, 13.6, 13.4, 5.9 and 12.0 ha in the Łabowiec, Łopuszna, Oszast, Uhryń and Żarnówka reserves, respectively. In total, about 250 trees in the Łopuszna, Oszast and Żarnówka reserves, and about 150 trees in the Łabowiec and Uhryń reserves, were preselected.
In 2017, pith cores were extracted from the selected trees using a Pressler drill and their species and dbh registered. The cores (one per tree) were taken upslope at a height of 1.3 m. All cores were taken upslope to facilitate the coring of dead trees frequently lying on the ground in the slope direction. Due to extensive internal trunk decay, some of the cores were not suitable for further processing. Similarly, some cores did not represent the whole lifespan of the cored trees, as missed tree pith or insertions of decaying wood did not allow the collection of complete data. Finally, 93 and 62 useful cores from upper layer Norway spruces and silver firs were designated for further analyses. In the case of middle layer trees, the sample sizes were 35 and 28, respectively. The mean (min. –max.) dbh of the cored trees was as follows: upper layer firs 55 (41–106) cm, upper layer spruces 54 (40–96) cm, middle layer firs 31 (22–39) cm, middle layer spruces 31 (20–36) cm. A more detailed specification of the cored trees is provided in Online Resource, Tables S1 and S2.
Data analysis
The cores were scanned with a high-resolution scanner and tree-ring widths measured using CDendro software [CybisElektronik, Sweden]. The tree-ring series of dead trees were cross-dated by using marker rings of the cored live individuals which were growing in neighboring stands with a less rigorous protection regime (Lombardi et al. 2008). We estimated prior tree diameters and corresponding radial increments based on measured dbh, tree-ring width series and empirical relationships between dbh and bark thickness. To control for bias in the estimation of retrospective dbhs attributable to trunk asymmetry, the analysis included only trees for which differences between the dbh measured in the field and calculated as the sums of radial increments were less than ± 20%. The differences between the measured and calculated dbhs were scattered in proportion to the tree-ring widths.
A percent growth change method was used for identification of release signals in the tree-ring chronologies. For a given year the percent growth change was calculated as 100[(Mposterior − Mprior)/Mprior], where Mprior is the mean tree-ring width over the prior 10 years, and Mposterior is the mean tree-ring width over the posterior 10 years (Nowacki and Abrams 1997). The percent growth change was calculated for each year in the chronology, except for the first and last decade due to constraints in the formula. A release signal was assigned when all of the following criteria were met: (1) in the following year the respective value of the percent growth change exceeded 50%, (2) the widths of at least seven out of 10 posterior tree-rings were greater than the mean width of 10 prior tree-rings, (3) the period of increased growth lasted more than 10 years (i.e. the mean width of 10 prior tree-rings was smaller than the mean width calculated for at least 10 posterior tree-rings) and (4) the period between neighboring release signals was at least 10 years.
The structural features (Paluch et al. 2015, 2021) and reconstructed dynamics of the studied stands were similar (Jastrzębski and Paluch 2022), and preliminary analyses did not show substantial differences in the mean age, age span, growth rate and release frequency of species on the between-site level. The calculations conducted for Gorce, Oszast and Żarnówka, where Norway spruce and silver fir co-occur, led to similar conclusions regarding both species as those conducted for all the stands. Therefore, to fully utilize valuable information from the cored trees, we aggregated data for spruce and fir from the studied locations and regarded them as representative for montane, mixed-species old-growth forests in the study region. The analysis of life histories was conducted separately for upper and middle layer trees. We assumed that these groups differ with respect to size and social position, but also the kind of information they provide in the context of stand dynamics: life histories of individuals from the upper layer reflect features of winner trees of a confirmed status which had successfully recruited from the regeneration and lower stand layers to the main canopy. In contrast, the future of smaller individuals still remains unknown, and in particular, some features of their life histories may decrease their chances of reaching the main canopy. The life histories were characterized based on variables related to tree age, release events recorded in their increment chronologies, radial increment and basal area increment, as described in Table 2. In addition to the basic characteristics (mean, standard deviation, minimum, maximum) and distributions of the analyzed variables, we tested whether populations of fir and spruce differ from each other. Because in some cases the distributions were asymmetric, we used permutation tests (100,000 permutations) for testing the statistical significance of the differences between spruce and fir (Good 2013). We also calculated Pearson correlation coefficients and partial correlation coefficients between the selected life history traits, dbh, and tree age. As previously, we used permutation tests (100,000 permutations) for testing the statistical significance of the correlation coefficients (Good 2013).
Results
Tree age, growth and canopy access time
The breast height age (hereafter, age) ranged between 89 and 294 years in upper layer spruces and between 96 and 328 years in upper layer firs (Table 3). Spruces at an age of 160–200 years and firs at an age of 120–200 years represented the greatest proportion in the total number of cored upper layer trees (Fig. 2). The mean age was insignificantly higher in the upper layer spruces than in the upper layer firs (191 vs. 177 years, p = 0.069). Middle layer trees were only insignificantly younger than upper layer trees (Fig. 2). The age of middle layer spruces ranged between 58 and 239 years and that of middle layer firs between 66 and 192 years (Table 4). Although the mean age of middle layer spruces and firs did not statistically differ (149 vs. 150 years), we found a higher proportion of spruces among middle layer trees below 120 years of age (Fig. 2b).
Trees of comparable age strongly differed in dbhs and basal areas (Fig. 3). The age of upper layer trees was negatively related with their growth rate expressed by the average tree-ring width (r = − 0.59, p < 0.001 for firs and r = − 0.78, p < 0.001 for spruces, Fig. 4). The age of upper layer trees was also negatively related with the growth rate in the period prior to their first release (r = − 0.29, p < 0.001 for firs and r = − 0.56, p < 0.001 for spruces). We did not find a clear relationship between dbh and tree age in either upper layer spruces or upper layer firs (Fig. 4). Nevertheless, our analysis revealed a significant relationship between tree age and dbh in middle layer spruces (r = 0.53, p < 0.001) but not in middle layer firs (Table 5). Cored upper layer trees needed on average 150 years (spruces) and 137 years (firs) to grow from a height of 130 cm to a dbh of 40 cm and thus to enter the upper canopy layer (Table 3). The fastest growing spruces and firs reached a dbh of 40 cm in 76 and 78 years, respectively. Fast growing upper layer trees, i.e. those with greater average tree ring widths, were released for the first time at a greater dbh (r = 0.50, p < 0.001 for firs and r = 0.46, p < 0.001 for spruces, Online Resource, Fig. S1). Similarly, younger upper layer spruces were released for the first time at a greater dbh (r = − 0.35, p < 0.001) than older trees of this species. In the case of fir, this latter relationship was not found. The relationships between growth rate and tree dbh at the first release persisted (r = 0.22, p < 0.04 for firs and r = 0.48, p < 0.001 for spruces) when trees with the greatest average tree-ring widths (> 3 mm) were discarded from the analysis.
Release events
We revealed up to seven release signals in the increment chronologies of the upper layer trees (Fig. 5). Trees with two or three release signals were the most frequently represented. Release events were registered in different decades (Online Resource, Fig. S2). The percentage of individuals with more than one release event was greater among upper layer firs than among upper layer spruces (Fig. 5a). Similarly, the mean number of release events per 100 years was greater in firs than in spruces (1.7 vs. 1.3, p < 0.001) (Table 3, Online Resource, Fig. S3). We found this tendency in all three stands in which both species co-occurred (Łopuszna 1.5 vs 1.2; Oszast 1.3 vs 1.2; Żarnówka 1.8 vs 1.3). Before entry into the upper stand layer (i.e. reaching a dbh of 40 cm), spruces and firs experienced a similar number of release events (1.9 vs. 2.0, n.s.). As in the case of upper layer trees, release signals were more frequent in middle layer firs than in middle layer spruces (Fig. 5b, Table 4). The periods of growth acceleration were registered at different life stages, including the stage after entry into the upper stand layer (i.e. exceeding the dbh threshold of 40 cm) (Online Resource, Fig. S4).
Before the first release, upper layer spruces grew more slowly than upper layer firs (1.3 vs. 1.6 mm year−1, p = 0.006, Table 3) and middle layer spruces as slowly as middle layer firs (1.0 vs. 1.0 mm year−1, n.s., Table 4). In most cases, a negative increment trend was registered before the first release, i.e. tree-ring widths decreased (Online Resource, Fig. S5). The first release (starting from a height of 130 cm) occurred mostly before the tree attained a dbh of 5 cm (Online Resource, Fig. S6). We did not find a significant difference between mean dbh (15.3 vs. 14.7 cm, n.s.) and mean age (50 vs. 46 years, n.s.) at which upper layer spruces and firs were released for the first time. Nonetheless, at the first release middle layer spruces tended to have a greater dbh (11 vs. 8 cm, p = 0.08) and age (57 vs. 38 years, p = 0.03) than middle layer firs (Table 4).
In the majority of upper layer trees, the period of growth acceleration lasted no longer than 10 years (Fig. 6) and was not related to tree species (Tables 3 and 4). This period lasted more than 25 years in only 14% of spruces and 16% of firs. The length of this period tended to weakly increase with the dbh of upper layer firs (r = − 0.24, p < 0.001) and middle layer spruces (r = − 0.34, p = 0.03) (Table 5, Online Resource, Fig. S7).
The post-release growth intensification was permanent (i.e. did not decrease below the pre-release level before the next release) in 47% of releases registered in upper layer spruces and in 34% of releases registered in upper layer firs (Fig. 7a). A similar effect was also observed in middle layer trees (Fig. 7b). In the case of trees with periodical (not permanent) growth intensification, this period lasted between 28 and 38 years depending on the species and stand layer (Tables 3 and 4). The length of the period of post-release growth intensification tended to decrease with the dbh of spruces (upper layer trees: r = − 0.23, p < 0.001; middle layer trees: = − 0.23, p = 0.03) and firs (upper layer trees: r = − 0.22, p = 0.03; middle layer trees: = − 0.65, p < 0.001) (Online Resource, Fig. S8).
Release events considerably increased tree growth. In most cases the ratio of maximal post-release increment to pre-release increment ranged between two to four (Online Resource, Fig. S9), but in single cases the increment increased more than tenfold. The average growth reaction of upper layer spruces tended to be weaker than that of upper layer firs (4.2 vs. 5.0, p = 0.072). The ratio tended to decrease with tree dbh (spruce: r = − 0.22, p < 0.001; fir: r= − 0.23, p < 0.001, Online Resource, Fig. S10) but not with the tree age at which the release occurred (Table 5). The ratio of maximal post-release increment to pre-release increment was similar in middle layer spruces and firs (4.0 vs. 4.1, n.s.) (Table 4). The ratio was not related to the length of the growth acceleration period in spruce or fir.
Tree-ring widths and basal area increment
In upper layer spruces, the mean ring widths were smaller than those in upper layer firs (1.65 vs. 1.84 mm year−1, p = 0.065) (Table 3). The greater increment of firs was particularly visible in the 50–60 cm dbh class and among the most rapidly growing trees (Fig. 8a). Middle layer spruces and firs grew slower than upper layer trees (Fig. 8b). We did not find a significant difference in the mean ring width between middle layer spruces and firs (1.17 vs. 1.18 mm year−1, n.s.) (Table 4).
Radial increment of the slowest growing trees (below the 25th percentile), fastest growing trees (above the 75th percentile) and trees of moderate growth rate (between the 25th and 75th percentile) in a given dbh class. The enlarged marks indicate dbh classes for which significant differences between spruce and fir were found
In upper layer spruces, the widest tree-ring widths were registered at a smaller dbh than in upper layer firs (28.4 vs. 35.6 cm, p = 0.019) (Table 3). The highest percentage of upper layer spruces (39%) showed the maximal radial increment at dbhs below 20 cm, whereas in upper layer firs, the maximal increment was most frequently registered in the 40–60 cm dbh class (Online Resource, Fig. S11). Variation in tree-ring widths (expressed for the single increment series as the standard deviation to mean ratio) was smaller in upper layer trees than in middle layer trees (0.41 vs. 0.63, p < 0.001) but did not differ between spruces and firs from the same stand layer (Tables 3 and 4, Online Resource, Fig. S12). We also obtained similar results for the ratio of maximal to minimal tree-ring width (Tables 3 and 4).
The average basal area increment increased with tree dbh, and we found no culmination of this characteristic in the analyzed dbh range (Fig. 9). In our data set trees entered the upper canopy (i.e. reached a dbh of 40 cm) between 1860 and 2010. The current basal area increment of trees of comparable social status (dbhs between 40 and 60 cm) changed over time; the increment of spruces and firs was smallest between 1955 and 1985 and greatest at the turn of the nineteenth and twentieth century (Online Resource, Fig. S13). When the current basal area increment during residence time in the 40–60 cm dbh class was analyzed and the effect of dbh was excluded by the calculation of partial correlation coefficients, we found a weak negative effect of tree age (Table 5). This means that the growth of older trees tended to be less than that of younger trees. On average, the current basal area increment decreased by about 5 cm2 per 100 years, and this effect did not differ significantly between fir and spruce (Fig. 10). Because of the small sample size of live trees, we could not conduct this analysis for trees of larger dbh.
In 54% of spruces and 67% of firs, the current basal area increment culminated during the last 5% of their lifetime and in 75% of spruces and 79% of firs during the last 10% of their lifetime (Online Resource, Fig. S14). This relation persisted regardless of whether only dead or live trees were considered in the analysis. At the age of the culmination of current basal area increment, upper layer spruces reached 87% and upper layer firs 86% of their terminal dbh, on average (Table 3). A considerable fraction of the oldest and largest trees reached the maximal current basal area increment at their terminal age or dbh (Fig. 11). We found no significant relationship between age or dbh and relative dbh at the culmination of the basal area increment.
During the last 20 years (1996–2015), 48% of live upper layer spruces showed a negative growth trend, i.e. tree-ring widths tended to decrease over time (Online Resource, Fig. S15). In upper layer firs the proportion of such trees was only 14%. The values of a slope coefficient characterizing the change in ring widths during 1996–2016 were substantially smaller in live upper layer spruces than in live upper layer firs (0.007 vs. 0.081, p < 0.001) (Table 3). In live upper layer firs (but not spruces), the positive growth trend tended to intensify with tree dbh, but because of the small sample size, we could not confirm this relationship statistically (Table 5, Online Resource, Fig. S16).
Discussion
Tree growth under gap-phase dynamics
Diameter increment is a very sensitive indicator of growth conditions and is frequently used to investigate competitive relationships (Ramseier and Weiner 2006). The obtained results fully corroborate our hypothesis that under the gap-phase dynamics typical for such forests, the pattern of radial increment of trees is not unimodal but usually includes alternate periods of slower and faster growth. The complex growth trajectories blurred the relationship between tree age and dbh, and trees of the same breast height age might differ in dbh by more than 50 cm. A similarly loose relationship was also found in managed single-tree selection forests with silver fir and Norway spruce (Schütz 2001). We revealed a negative relationship between tree age and average growth rate (here expressed by the average tree-ring width over an entire lifetime) and a positive relationship between average growth rate and the dbh at which a tree was released for the first time. This implies that young trees growing in the upper layer were growing faster, probably in less competitive surroundings, and the first release occurred later in their life-history, already at larger dbhs. Only about 10% of the increment series showed regular growth, without visible periods of growth suppression or acceleration. It may be assumed that such trees, starting from the age at which they had reached a height of 130 cm (at which we took the cores), grew under relatively constant pressure from competing neighbor trees, although not necessarily in large canopy gaps. In the upper layer trees, we registered mostly two or three release signals, and the increment series of 9% of spruces and 15% of firs contained four to seven release signals. We assume that the majority of the revealed periods of abrupt growth acceleration are associated with disturbances which caused the death of one or several trees competing with the individual in focus.
The course of post-release growth of trees reflects variation in growth conditions and gap sizes created by disturbances. It may be assumed that the improvement of growth conditions triggered by a severe disturbance is more lasting than that brought about by the death of a single less important competitor. In more than half of the increment series (53% of upper layer spruces and 66% of upper layer firs), the increment decreased below the pre-release level before the successive release events occurred. This suggests that the growth acceleration was triggered by small disturbances which did not lead to a definitive release with permanent growth enhancement. We also found a considerable portion of trees in which the post-release radial increment did not decrease below the pre-release level (47% of upper layer spruces and 34% of upper layer firs). Such an effect seems attributable to more severe disturbances, which killed all the important competitors in the neighborhood of the released individual, but also to a situation with a re-occurrence of a disturbance before the radial increment decreased below the pre-release level.
The growth reaction to release may depend on social status and prior growth conditions (Metslaid et al. 2007; Puettmann et al. 2009). The release events are also frequently associated with damage to the released individual caused by falling trunks or boughs of uprooted or snapped neighbors. Baral et al. (2016) showed that the likelihood of a tree possessing stem damage increases with the length of suppression but decreases with increasing residual stand basal area. In accordance with former reports for shade-tolerant species (Wright et al. 2000), we did not find tree age as an important factor in the growth response of silver fir and Norway spruce following release. It should be stressed, however, that the majority of upper canopy trees experienced their first release relatively early (at a dbh of less than 10 cm), and only 17% of spruces and 28% of firs later, at a dbh greater than 20 cm. Moreover, the release events before the trees entered the upper stand layer were relatively frequent (about two at a mean canopy access time of 130–150 years), thus periods of durable growth suppression were relatively rare.
Delays in response to release reflect physiological and morphological adjustments following overstory removal, and it can take several years after release for secondary (diameter) growth to occur on a tree (Youngblood 1991). We registered maximal dbh growth not later than 15 years after the release event in more than half of trees. An insignificantly shorter response lag (5–10 years) was reported by Puettmann et al. (2009) in their study in mature silver fir stands subjected to irregular shelterwood cuttings. Elkin et al. (2015) and Sohn et al. (2016) also reported similarly short intervals (5–10 years) in which Norway spruces benefited from thinning. In our study the growth reaction to release tended to decrease with tree size. A weaker reaction of trees of a dominant social status to release from competition is well documented (Freist 1962; Jones et al. 2009; Puettmann et al. 2009). This effect seems attributable to the lower density of the upper stand layer, where trees frequently grow in less competitive surroundings, increased transpirational stress and slower physiological adaptation of needles to high radiation levels (Renninger et al. 2007).
An unimodally shaped curve is a commonly accepted model of the current annual growth of a tree (Assmann 1971, Wenk et al. 1990; Thomas 2013). Lower stem increments often precede tree death (Das et al. 2016; Cailleret et al. 2017). The culmination of current basal area (eventually volume/mass) increment may be viewed as a natural boundary between the optimum and aging stage in the ontogenic development of a tree. Our analyses revealed that the studied individuals from the upper stand layer achieved their maximal basal area increment at the final stage of their life history irrespective of age or dbh. In the case of live trees, this might simply mean that the factual culmination had not yet been achieved. We found this growth pattern not only in small and mid-aged but also in large and old trees (at an age of above 200 years). On this basis it may be assumed that a considerable fraction of upper layer trees may be killed by natural disturbances before their increment reaches the culmination point and before they reach the ontogenic stage of aging. In fact, in former studies in Western Carpathian old-growth forests (Paluch et al. 2015) among dead trees from the upper stand layer we found a dominant fraction of trees of relatively small dbhs, close to 50 cm, which most probably had not yet reached the senile phase of their life. These results are in line with findings that the volume growth of trees may persist at a high level until advanced ages, far beyond the usual rotation age (Korpel 1995; Sillet et al. 2010; Castagneri et al. 2013; Stephenson et al. 2014; Sillett et al. 2015; Piovesan et al. 2019). Pretzsch (2020) documented a mean mass/volume culmination age of about 230 years for old Norway spruces (much higher for sessile oaks and European beeches) and concluded that any growth decrease by the ratio between assimilating surface area of a tree and respiring volume or mass may happen, but probably far beyond the tree ages or tree size assumed so far. It is more likely that healthy predominant or dominant trees continue to grow until damaged by mechanical disturbances or pathogens instead of continuously decreasing in growth due to the prevalence of respiration. In this context, it is worth adding that among dead trees from the upper stand layer—in the studied old-growth forests killed exclusively by natural disturbances—we frequently found individuals with a progressive growth trend visible in the ring widths during 1996–2015, that is in the terminal stage of their life. Obviously, because stem volume and mass increase approximately proportional to the third power of tree dbh, this means that the volume growth had to grow much more.
The long-lasting growth of the studied trees may also be partly caused by environmental changes such as eutrophication, increased temperature and extension of the growing season (Pretzsch et al. 2014). There are abundant literature reports on enhanced tree growth over the last decades across Europe linked to climatic and anthropogenic factors (Pretzsch et al. 2014). In some regions this impact was concurrent with decreasing air pollution which first triggered a strong growth depression in forests from the 1960s to the mid of 1980s and subsequently brought about a rapid revitalization and growth enhancement of trees (Bošeľa et al. 2014; Wertz et al. 2014). Nonetheless, the capability of old trees with often complex life histories to effectively respond to these changes at an advanced age may be seen as a testimony to their growth plasticity and adaptability to a changing environment.
The growth trends of spruces and firs differed during last two decades. In the period 1996–2015 the majority of spruces showed a regressive (or neutral) growth trend, whereas the majority of firs showed a progressive growth trend. The studied old-growth forests are located in the middle (Łabowiec, Uhryń) and upper part of the lower montane belt (Łopuszna, Oszast, Żarnówka), where increasing summer temperatures at a constant precipitation (Wypych et al. 2018) caused the positive growth reaction of fir (Wertz et al. 2014; Wilczyński and Szymański 2015) and to a lesser extent also of spruce (Savva et al. 2006; Wilczyński et al. 2015). Nonetheless, at low and intermediate elevations increasing summer temperatures and deepening water stress may induce a negative growth response in Norway spruce as its sensitivity to these factors is strongly site-dependent (Levanič et al. 2009; Sidor et al. 2015).
Differences in the life histories of spruces and firs
Light is a key driver shaping species-specific growth trajectories and allocation patterns (Brüllhardt et al. 2020). Fir and spruce demonstrate similar plasticity of crown, shoot and needle morphology under changing light conditions (Grassi and Giannini 2005), and trees of both species are capable of surviving under low light intensities (Lundqvist and Nilson 2007; Diaci and Firm 2011; Lin et al. 2012; Vencurik et al. 2020). Nonetheless, numerous studies have shown that spruce has a greater ability to utilize high-light conditions compared to fir (Grassi and Bagnaresi 2001) and that in conditions close to full light, young spruces grow faster (Brunner 1993; Brunner and Huss 1994) or have similar growth rates as fir (Stanciou and O’Hara 2006). In contrast, fir usually demonstrates a greater ability to maintain a positive carbon balance at low light intensities as compared to spruce (Aussenac 2000). Several studies have found that the probability of Norway spruce establishment diminished with increasing overstory density (Schweiger and Sterba 1997; Lin et al. 2012; Paluch et al. 2021). In smaller gaps or under overstoreys of moderate to high densities, the height growth of spruces is lower than that of firs (Golser and Hasenauer 1997; Stancioiu and O’Hara 2006; Vencurik et al. 2020). Dănescu et al. (2018) demonstrated that even rapid group-shelterwood cutting regimes with complete canopy removal within two decades still favored fir regeneration.
Based on the lower shade tolerance of spruce, in the increment series of this species we expected to find evidence of early release, more rapid growth at a young age, more linear growth with fewer release signals and a smaller canopy access time compared to fir. However, the obtained results do not corroborate the majority of our assumptions. Statistically confirmed differences were found only for several analyzed variables, and the relations between spruce and fir were not always concordant with our initial conjectures. Although upper layer spruces indeed experienced fewer releases (1.3 vs. 1.7 per 100 years), attained culmination of radial increment at smaller dbhs (28 vs. 36 cm) and had a weaker response to releases (i.e. the ratios of their maximal post-release to prior-release radial increment were smaller than those in firs, 4.2 vs. 5.0), they entered the upper canopy at a similar age as firs (145 vs. 134 years) and grew just as slowly or even more slowly than firs of comparable dbh. Moreover, we did not find evidence that upper layer spruces experienced their first release at a smaller dbh (15 vs. 17 cm) and as younger individuals compared to fir (50 vs. 46 years), or that they grew faster than firs before the first release (1.3 vs. 1.6 mm per year). Similarly, we were unable to prove that the tree-ring series of spruces were less variable (i.e. had a lower standard deviation to mean ratio) than those of firs (0.41 vs. 0.40).
Variability in the light microclimate in canopy gaps allows the establishment and growth of tree species with diverse ecological requirements (Gravel et al. 2010; Čater et al. 2014). Nonetheless, the picture which emerges from our study is that the mechanism of site partitioning, i.e. that spruces survive in specific, less shaded locations, was not a sine qua non condition for the persistence of this species in the studied stands. It seems rather that the populations of spruce and fir had been growing in a similar environment in mixed-species stands with beech. The secondary role of the site partitioning mechanism suggested by our results raises the question why fir and beech did not entirely outcompete spruce during the stand development. To answer this question several non-mutually exclusive hypothetical mechanisms may be proposed. The first involves browsing by ungulates as an important biotic driver of regeneration success (Kupferschmid et al. 2015). Fir is strongly preferred by ungulates as compared to spruce and this factor can shift the composition of fir-spruce regeneration cohorts towards spruce predominance (Mosandl and El Kateb 1988). Nonetheless, the historical data indicate that the population of deer was sparse at the beginning of the twentieth century (presumably also earlier) and damage to regeneration were of little importance then.
The second hypothesis is that in the past the overstory was less dense than at present and the competitive predominance of fir and beech in relation to spruce was smaller. Although the reconstruction of past disturbances since the mid nineteenth century allowed the exclusion of large-scale, severe disturbances (Jastrzębski and Paluch 2022), structural changes in the former period are poorly recognized. It is worth noting that in our stands the retrospective radial increment of upper layer trees was relatively high and equalized between dbh classes (see Fig. 8). In multi-aged managed silver fir forests with single-tree selection, the dbh increment typically increases with tree dbh at least up to a diameter of 40 cm. For example, in site conditions comparable to the studied stands, at a stand basal area between 40 and 45 m2 ha−1, the mean dbh increment of trees of dbh 7, 15 and 25 cm is about 0.5, 1.8 and 2.8 mm per year on average, respectively (Kubiński 2019). At present, a similar pattern of tree growth is also registered on permanent research plots in the studied old-growth forests (Paluch et al. 2022). Thus, in comparison to the present conditions, the retrospective growth of the cored upper layer trees was faster, particularly at small diameters. This corroborates the hypothesis of a lower density of historical stands in the studied region. Moreover, the compilation of historical records, data from inventory plans, measurements on permanent research plots, and scientific reports from other locations in the Western Carpathians (Vrška et al. 2009; Jaworski 2016) strongly support the thesis that the species composition of the tree layer in the studied forests considerably altered over the last century, namely the percentage of conifer species decreased and the percentage of European beech increased. For example, in Żarnówka—a reserve with the best documented history in the Polish part of the Western Carpathians—the percentage of beech in the number of stems (dbh equal or greater than 7 cm) has increased from about 38 to 82% over the last century (Paluch et al. 2022). A vertically heterogeneous canopy formed by conifer species such as fir and spruce allows more lateral light to reach the understory (Ligot et al. 2016) and leads to a patchier distribution of soil resources that facilitates better uptake of water and nutrients by juvenile trees. In contrast, European beech has remarkable growth plasticity, an expansive crown, and mono-layer foliage distribution and is thus able to strongly overshadow the forest interior (Schröter et al. 2012; Bayer et al. 2013; Krůček et al. 2019). Hence, an increasing proportion of European beech in the stand species composition can negatively influence the survival and growth of spruces and firs. As a consequence, despite the improving climatic determinants of growth, conditions are less advantageous for young spruces and firs at present compared to several decades earlier, when European beech occurred at a considerably lower frequency.
The third hypothesis which may help explain the persistence of spruce takes into account climatic factors and assumes that the colder climate during the nineteenth century might favor spruce against fir and beech. The positive effect of climate warming on the growth of fir and beech at the upper range of their altitudinal occurrence has been shown in several reports (Dulamsuren et al. 2017; Hilmers et al. 2019). The comparison of average radial increments of spruces and firs from the Babia Gora National Park indicate that in the mid eighteenth century the diameter growth of the both species was more similar than at present (Szwagrzyk and Szewczyk 2001). The studied old-growth forests with spruces are located in the upper part of the lower montane belt (930–1140 m asl), and a shift in the mean annual temperature of 0.5–1.5 °C (or 100–300 m in altitude) causes a change in natural species composition from mixed-species stands to pure spruce forests. Therefore, given the natural inertia of stand species composition resulting from the longevity of trees and spatio-temporal heterogeneity intrinsic to natural stand dynamics, climatic fluctuations indeed might effectively counteract the competitive exclusion of spruce.
Conclusions
Our study shows that in natural multi-aged stands driven by gap-phase dynamics, the course of radial increment of Norway spruces and silver firs is governed by social status, here represented by tree dbh, and disturbances, which induce sequences of accelerated and suppressed growth. Within the range encompassed by our data, we found no significant relationships between tree age and response to release events and only a weak negative relationship between the age and current basal area increment of live upper canopy trees. Moreover, a late culmination of current basal area increment or its lack in the lifespan of many individuals suggests that in natural conditions trees are frequently killed by extraneous factors before they reach the aging phase characterized by decreasing growth. Thus, in spruces and firs growing in old-growth forests, age is a secondary driver of tree development. This characteristic may be viewed as an important adaptation to an environment driven by gap-phase dynamics developed during the evolution of shade tolerant late-seral species. Further research is needed to gain insight into the ecological and evolutionary importance of changing growth patterns of shade tolerant trees in managed forest, where the focus is frequently on enhancing tree growth and avoiding suppression periods.
In contrast to our hypotheses, we found only insignificant differences between the life histories of firs and spruces. This calls into question site partitioning as a mechanism conditioning the persistence of Norway spruce in mixtures of more shade-tolerant and competitive species. Rather, the persistence of spruce is attributable to the more severe climate in the nineteenth century, temporal inertia of species composition, lower stand densities and lower percentage of beech in the past.
Although our study documents the considerable growth plasticity of Norway spruce, we also realize that the growth conditions of this species are dramatically affected by climate change and that the general growth pattern and amplitude of the growth reaction to competitive suppression known from the past may not be directly transferred to the present and future conditions. In contrast, silver fir responds well to climatic change, replaces spruce in many forest ecosystems in Central Europe and is commonly regarded as a keystone species for adaptive forest management promoting species diversity and complex stand structures. In this respect our study provides evidence that in site conditions similar to those in our research, even trees with complex life histories and long periods of past growth suppression can respond well to release and continue growing without clear signs of earlier senescence. This should encourage the integration of old trees and individuals of suppressed growth into silvicultural treatments aimed not only as a measure of protection of biodiversity but also effective stand conversion. Further study is needed in the area of the internal structure, external allometry and morphology of suppressed trees as the main drivers of their growth reaction to release.
References
Abrams MD, Orwig DA (1996) A 300-year history of disturbance and canopy recruitment for co-occurring white pine and hemlock on the Allegheny Plateau, USA. J Ecol 84:353–363. https://doi.org/10.2307/2261198
Amos-Binks LJ, MacLean DA (2016) The influence of natural disturbances on developmental patterns in Acadian mixedwood forests from 1946 to 2008. Dendrochronologia 37:9–16. https://doi.org/10.1016/j.dendro.2015.11.002
Assmann E (1961) Waldertragskunde. BLV Verlagsgesellschaft, München
Aussenac G (2000) Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silviculture. Ann For Sci 57:287–301. https://doi.org/10.1051/forest:2000119
Backhaus S, Kreyling J, Grant K, Beierkuhnlein C, Walter J, Jentsch A (2014) Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems 17:1068–1081. https://doi.org/10.1007/s10021-014-9781-5
Baral SK, Danyagri G, Girouard M, Hébert F, Pelletier G (2016) Effects of suppression history on growth response and stem quality of extant northern hardwoods following partial harvests. For Ecol Manage 372:236–246. https://doi.org/10.1016/j.foreco.2016.04.023
Bayer D, Seifert S, Pretzsch H (2013) Structural crown properties of Norway spruce (Picea abies L. Karst.) and European beech (Fagus sylvatica L.) in mixed versus pure stands revealed by terrestrial laser scanning. Trees Struct Funct 27:1035–1047. https://doi.org/10.1007/s00468-013-0854-4
Bebber DP, Thomas SC, Cole WG, Balsillie D (2004) Diameter increment in mature eastern white pine Pinus strobus L. following partial harvest of old-growth stands in Ontario. Canada Trees Struct Funct 18:29–34. https://doi.org/10.1007/s00468-003-0274-y
Bigler C, Veblen TT (2009) Increased early growth rates decrease longevities of conifers in subalpine forests. Oikos 118:1130–1138. https://doi.org/10.1111/j.1600-0706.2009.17592.x
Bošeľa M, Petráš R, Sitková Z, Priwitzer T, Pajtík J, Hlavatá H, Sedmák R, Tobin B (2014) Possible causes of the recent rapid increase in the radial increment of silver fir in the Western Carpathians. Environ Poll 184:211–221. https://doi.org/10.1016/j.envpol.2013.08.036
Brüllhardt M, Rotach P, Bigler C, Nötzli M, Bugmann H (2020) Growth and resource allocation of juvenile European beech and sycamore maple along light availability gradients in uneven-aged forests. For Ecol Manage 474:118314. https://doi.org/10.1016/j.foreco.2020.118314
Brunner A (1993) Die Entwicklung von Bergmischwaldkulturen in den Chiemgauer Alpen und eine Methodenstudie zur ökologischen Lichtmessung im Wald. Forstliche Forschungsberichte, München, p 128
Brunner A, Huss J (1994) Die Entwicklung von Bergmischwaldkulturen in den Chiemgauer Alpen. Forstw Cbl 113:194–203
Cailleret M, Jansen S, Robert EMR et al (2017) A synthesis of radial growth patterns preceding tree mortality. Glob Change Biol 23:1675–1690. https://doi.org/10.1111/gcb.13535
Camarero JJ, Gazol A, Sangüesa-Barreda G, Cantero A, Sánchez-Salguero R, Sánchez-Miranda A, Ibáñez R (2018) Forest growth responses to drought at short-and long-term scales in Spain: squeezing the stress memory from tree rings. Front Ecol Evol 6:9. https://doi.org/10.3389/fevo.2018.00009
Castagneri D, Storaunet KO, Rolstad J (2013) Age and growth patterns of old Norway spruce trees in Trillemarka forest, Norway. Scand J for Res 28:232–240. https://doi.org/10.1080/02827581.2012.724082
Čater M, Diaci J, Roženbergar D (2014) Gap size and position influence variable response of Fagus sylvatica L. and Abies alba Mill. For Ecol Manage 325:128–135. https://doi.org/10.1016/j.foreco.2014.04.001
Claveau Y, Messier C, Comeau PG, Coates KD (2002) Growth and crown morphological responses of boreal conifer seedlings and saplings with contrasting shade tolerance to a gradient of light and height. Can J for Res 32:458–468. https://doi.org/10.1139/x01-220
Dănescu A, Kohnle U, Bauhus J, Weiskittel A, Albrecht AT (2018) Long-term development of natural regeneration in irregular, mixed stands of silver fir and Norway spruce. For Ecol Manage 430:105–116. https://doi.org/10.1016/j.foreco.2018.07.055
Das AJ, Stephenson NL, Davis KP (2016) Why do trees die? Characterizing the drivers of background tree mortality. Ecology 97:2616–2627. https://doi.org/10.1002/ecy.1497
Di Filippo A, Biondi F, Piovesan G, Ziaco E (2017) Tree ring-based metrics for assessing old-growth forest naturalness. J Appl Ecol 54:737–749. https://doi.org/10.1111/1365-2664.12793
Di Filippo A, Pederson N, Baliva M, Brunetti M, Dinella A, Kitamura K, Knapp HD, Schirone B, Piovesan G (2015) The longevity of broadleaf deciduous trees in Northern Hemisphere temperate forests: insights from tree-ring series. Front Ecol Evol 3:347. https://doi.org/10.3389/fevo.2015.00046
Diaci J, Firm D (2011) Long-term dynamics of a mixed conifer stand in Slovenia managed with a farmer selection system. For Ecol Manage 262:931–939. https://doi.org/10.1016/j.foreco.2011.05.024
Domec JC, King JS, Ward E et al (2015) Conversion of natural forests to managed forest plantations decreases tree resistance to prolonged droughts. For Ecol Manage 355:58–71. https://doi.org/10.1016/j.foreco.2015.04.012
Dulamsuren C, Hauck M, Kopp G, Ruff M, Leuschner C (2017) European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (SW Germany). Trees Struct Funct 31:673–686. https://doi.org/10.1007/s00468-016-1499-x
Elkin C, Giuggiola A, Rigling A, Bugmann H (2015) Short-and long-term efficacy of forest thinning to mitigate drought impacts in mountain forests in the European Alps. Ecol Appl 25:1083–1098. https://doi.org/10.1890/14-0690.1
Emborg J (2007) Suppression and release during canopy recruitment in Fagus sylvatica and Fraxinus excelsior - a dendro-ecological study of natural growth patterns and competition. Ecol Bull 52:53–67
Firm D, Nagel TA, Diaci J (2009) Disturbance history and dynamics of an old-growth mixed species mountain forest in the Slovenian Alps. For Ecol Manage 257:1893–1901. https://doi.org/10.1016/j.foreco.2008.09.034
Frankovič M, Janda P, Mikoláš M et al (2021) Natural dynamics of temperate mountain beech-dominated primary forests in Central Europe. For Ecol Manage 479. https://doi.org/10.1016/j.foreco.2020.118522
Freist H (1962) Untersuchungen über den Lichtungszuwachs der Rotbuche und seine Ausnützung im Forstbetrieb. Beih Forstwiss Cbl 17
Golser M, Hasenauer H (1997) Predicting juvenile tree height growth in uneven-aged mixed species stands in Austria. For Ecol Manage 97:133–146. https://doi.org/10.1016/S0378-1127(97)00094-7
Good P (2013) Permutation tests: a practical guide to resampling methods for testing hypotheses. Springer, Cham
Grassi G, Bagnaresi U (2001) Foliar morphological and physiological plasticity in Picea abies and Abies alba saplings along a natural light gradient. Tree Physiol 21:959–967. https://doi.org/10.1093/treephys/21.12-13.959
Grassi G, Giannini R (2005) Influence of light and competition on crown and shoot morphological parameters of Norway spruce and silver fir saplings. Ann For Sci 62:269–274. https://doi.org/10.1051/forest:2005019
Grassi G, Minotta G, Tonon G, Bagnaresi U (2004) Dynamics of Norway spruce and silver fir natural regeneration in a mixed stand under uneven-aged management. Can J For Res 34:141–149. https://doi.org/10.1139/x03-197
Gravel D, Canham CD, Beaudet M, Messier C (2010) Shade tolerance, canopy gaps and mechanisms of coexistence of forest trees. Oikos 119:475–484. https://doi.org/10.1111/j.1600-0706.2009.17441.x
Gray AN, Spies TA, Pabst RJ (2012) Canopy gaps affect long-term patterns of tree growth and mortality in mature and old-growth forests in the Pacific Northwest. For Ecol Manage 281:111–120. https://doi.org/10.1016/j.foreco.2012.06.03
Grundner F, Schwappach A (1952) Massentaffeln zur Bestimmung des Holzgehaltes stehender Waldbäume und Waldbestände. Parey, Berlin
Hilmers T, Avdagić A, Bartkowicz L, Bielak K, Binder F, Bončina A, Jaworski A (2019) The productivity of mixed mountain forests comprised of Fagus sylvatica, Picea abies, and Abies alba across Europe. Forestry 92:512–522. https://doi.org/10.1093/forestry/cpz035
Hynynen J, Eerikäinen K, Mäkinen H, Valkonen S (2019) Growth response to cuttings in Norway spruce stands under even-aged and uneven-aged management. For Ecol Manage 437:314–323. https://doi.org/10.1016/j.foreco.2018.12.032
Jastrzębski R, Paluch J (2022) The spatio-temporal pattern of release signals and tree growth in Fagus-Abies-Picea old-growth forests reveals unsteady gap-phase dynamics. For Ecol Manage 503:119743. https://doi.org/10.1016/j.foreco.2021.119743
Jaworski A (1979) Wzrost i żywotność podrostu jodły (Abies alba Mill.) w drzewostanach o różnej strukturze na przykładzie wybranych powierzchni w Karpatach i Sudetach. Acta Agr Et Silv Ser Silv 18:61–79
Jaworski A (2016) Dolnoreglowe lasy o charakterze pierwotnym w Babiogórskim Parku Narodowym (lata 1930–2006). Wydawnictwo Uniwersytetu Rolniczego w Krakowie, Kraków
Jones TA, Domke GM, Thomas SC (2009) Canopy tree growth responses following selection harvest in seven species varying in shade tolerance. Can J For Res 39:430–440. https://doi.org/10.1139/X08-186
Kaufmann S, Hauck M, Leuschner C (2018) Effects of natural forest dynamics on vascular plant, bryophyte, and lichen diversity in primeval Fagus sylvatica forests and comparison with production forests. J Ecol 106:2421–2434. https://doi.org/10.1111/1365-2745.12981
Kneeshaw DD, Williams H, Nikinmaa E, Messier C (2002) Patterns of above- and below-ground response of understory conifer release 6 years after partial cutting. Can J For Res 32:255–265. https://doi.org/10.1139/x01-190
Kohlermann L (1950) Untersuchungen über die Windverbreitung der Früchte und Samen mitteleuropäischer Waldbäume. Forstwiss Cbl 69:606–624
Korpel Š (1995) Die Urwälder der Westkarpaten. Gustav Fisher Verlag, Stuttgart
Král K, Valtera M, Janik D, Šamonil P, Vrška T (2014) Spatial variability of general stand characteristics in central European beech—dominated natural stands—effects of scale. For Ecol Manage 328:353–364. https://doi.org/10.1016/j.foreco.2014.06.034
Krůček M, Trochta J, Cibulka M, Král K (2019) Beyond the cones: How crown shape plasticity alters aboveground competition for space and light—evidence from terrestrial laser scanning. Agric For Meteor 264:188–199. https://doi.org/10.1016/j.agrformet.2018.09.016
Kubiński R (2019) Charakterystyka przyrostu grubości drzew w jodłowych drzewostanach przerębowych wzrastających na siedliskach wyżynnych i górskich Krainy Karpackiej. Master thesis, Faculty of Forestry, University of Agriculture, Kraków
Kupferschmid AD, Wasem U, Bugmann H (2015) Browsing regime and growth response of Abies alba saplings planted along light gradients. Eur J For Res 134:75–87. https://doi.org/10.1007/s10342-014-0834-2
Latham P, Tappeiner J (2002) Response of old-growth conifers to reduction in stand density in western Oregon forests. Tree Physiol 22:137–146. https://doi.org/10.1093/treephys/22.2-3.137
Leuschner C, Ellenberg H (2017) Ecology of Central European forests. Springer International Publishing, Cham, Vegetation ecology of Central Europe
Levanič T, Gričar J, Gagen M et al (2009) The climate sensitivity of Norway spruce [Picea abies (L.) Karst.] in the southeastern European Alps. Trees Struct Funct 23:169–180. https://doi.org/10.1007/s00468-008-0265-0
Ligot G, Ameztegui A, Courbaud B, Coll L, Kneeshaw D (2016) Tree light capture and spatial variability of understory light increase with species mixing and tree size heterogeneity. Can J For Res 46:968–977. https://doi.org/10.1139/cjfr-2016-0061
Lin CJ, Laiho O, Lähde E (2012) Norway spruce (Picea abies L.) regeneration and growth of understory trees under single-tree selection silviculture in Finland. Eur J For Res 131:683–691. https://doi.org/10.1007/s10342-011-0541-1
Lindenmayer DB (2017) Conserving large old trees as small natural features. Biol Conserv 211:51–59. https://doi.org/10.1016/j.biocon.2016.11.012
Lombardi F, Cherubini P, Lasserre B, Tognetti R, Marchetti M (2008) Tree rings used to assess time since death of deadwood of different decay classes in beech and silver fir forests in the central Apennines (Molise, Italy). Can J For Res 38:821–833. https://doi.org/10.1139/X07-195
Lorimer CG, Dahir SE, Nordheim EV (2001) Tree mortality rates and longevity in mature and old-growth hemlock-hardwood forests. J Ecol 89:960–971. https://doi.org/10.1111/j.1365-2745.2001.00619.x
Lundqvist L, Nilson K (2007) Regeneration dynamics in an uneven-sized virgin Norway spruce forest in northern Sweden. Scand J For Res 22:304–309. https://doi.org/10.1080/02827580701479717
Metslaid M, Jõgiste K, Nikinmaa E, Moser WK, Porcar-Castell A (2007) Tree variables related to growth response and acclimation of advance regeneration of Norway spruce and other coniferous species after release. For Ecol Manage 250:56–63. https://doi.org/10.1016/j.foreco.2007.03.009
Moning Ch, Müller J (2009) Critical forest age thresholds for the diversity of lichens, mollusks and birds in beech (Fagus sylvatica L.) dominated forests. Ecol Indic 9:922–932. https://doi.org/10.1016/j.ecolind.2008.11.002
Morimoto J, Nakagawa K, Takano et al (2019) Comparison of vulnerability to catastrophic wind between Abies plantation forests and natural mixed forests in northern Japan. Forestry 92:436–443. https://doi.org/10.1093/forestry/cpy045
Mosandl R, El Kateb H (1988) Die verjüngung gemischter Bergwälder — praktische konsequenzen aus 10-jähriger Untersuchungsarbeit. Forstwiss Cbl 107:2–13
Nagel TA, Svoboda M, Kobal M (2014) Disturbance, life history traits, and dynamics in an old-growth forest landscape of southeastern Europe. Ecol Appl 24:663–679. https://doi.org/10.1890/13-0632.1
Nowacki GJ, Abrams MD (1997) Radial-growth averaging criteria for reconstructing disturbance histories from presettlement-origin oaks. Ecol Monogr 67:225–249. https://doi.org/10.1890/0012-9615(1997)067[0225:RGACFR]2.0.CO;2
Ogle K, Barber JJ, Barron-Gafford GA, Bentley LP, Young JM, Huxman TE, Loik ME, Tissue DT (2015) Quantifying ecological memory in plant and ecosystem processes. Ecol Lett 18:221–235. https://doi.org/10.1111/ele.12399
Paluch J (2007) The spatial pattern of a natural European beech (Fagus sylvatica L.) - silver fir (Abies alba Mill.) forest: a patch-mosaic perspective. For Ecol Manage 253:161–170. https://doi.org/10.1016/j.foreco.2007.07.013
Paluch J (2021) The stochastic backward shifts model better corresponds to the fine-scale structural heterogeneity of old-growth Abies-Fagus-Picea forests than the ontogenic life cycle model. For Ecol Manage 118978. https://doi.org/10.1016/j.foreco.2021.118978
Paluch J, Keren S, Govedar Z (2021) The Dinaric Mountains versus the Western Carpathians: is structural heterogeneity similar in close-to-primeval Abies-Picea-Fagus forests? Eur J For Res 140:209–225. https://doi.org/10.1007/s10342-020-01325-0
Paluch J, Kołodziej Z, Pach M, Jastrzębski R (2015) Spatial variability of close-to primeval Fagus-Abies-Picea forests in the Western Carpathians (Central Europe): a step towards a generalised pattern. Eur J For Res 134:235–246. https://doi.org/10.1007/s10342-014-0846-y
Paluch J, Szewczyk J, Muter E, Szwagrzyk J (2022) Struktura i dynamika lasów dolnoreglowych. In: Szwagrzyk J (ed) Lasy Babiogórskiego Parku Narodowego. Babiogórski Park Narodowy, Zawoja, pp 115–186
Parobeková Z, Pittner J, Kucbel S, Saniga M, Filípek M, Sedmáková D, Vencurik J, Jaloviar P (2018) Structural diversity in a mixed spruce-fir-beech old-growth forest remnant of the Western Carpathians. Forests 9:379. https://doi.org/10.3390/f9070379
Paszyński J, Niedźwiedź T (1999) Klimat. In: Starkel L (ed) Geografia Polski. Środowisko przyrodnicze. PWN, Warszawa, pp 288–343
Pedersen BS, Howard JL (2004) The influence of canopy gaps on overstory tree and forest growth rates in a mature mixed-age, mixed-species forest. For Ecol Manage 196:351–366. https://doi.org/10.1016/j.foreco.2004.03.031
Piovesan G, Biondi F, Baliva M, De Vivo G, Marchianò V, Schettino A, Di Filippo A (2019) Lessons from the wild: slow but increasing long-term growth allows for maximum longevity in European beech. Ecology 100(9):e02737. https://doi.org/10.1002/ecy.2737
Piovesan G, Biondi F (2021) On tree longevity. New Phytol 231:1318–1337. https://doi.org/10.1111/nph.17148
Pretzsch H (2020) The course of tree growth. Theory Reality For Ecol Manage 478:118508. https://doi.org/10.1016/j.foreco.2020.118508
Pretzsch H (2022) The emergent past: past natural and human disturbances of trees can reduce their present resistance to drought stress. Eur J For Res 141:87–104. https://doi.org/10.1007/s10342-021-01422-8
Pretzsch H, Biber P, Schütze G, Uhl E, Rötzer T (2014) Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat Commun 5:4967. https://doi.org/10.1038/ncomms5967
Puettmann KJ, D’Amato AW, Kohnle U, Bauhus J (2009) Individual-tree growth dynamics of mature Abies alba during repeated irregular group shelterwood (Femelschlag) cuttings. Can J For Res 39:2437–2449. https://doi.org/10.1139/X09-158
Ramseier D, Weiner J (2006) Competitive effect is a linear function of neighbour biomass in experimental populations of Kochia scoparia. J Ecol 94:305–309. https://doi.org/10.1111/j.1365-2745.2005.01082.x
Raymond P, Bédard S, Roy V, Larouche C, Tremblay S (2009) The irregular shelterwood system: review, classification, and potential application to forests affected by partial disturbances. J For 107:405–413. https://doi.org/10.1093/jof/107.8.405
Renninger HJ, Meinzer FC, Gartner BL (2007) Hydraulic architecture and photosynthetic capacity as constraints on release from suppression in Douglas-fir and western hemlock. Tree Physiol 27:33–42. https://doi.org/10.1093/treephys/27.1.33
Roberts SD, Harrington CA (2008) Individual tree growth response to variable-density thinning in coastal Pacific Northwest forests. For Ecol Manage 255:2771–2781. https://doi.org/10.1016/j.foreco.2008.01.043
Rozenbergar D, Mikac S, Anić I, Diaci J (2007) Gap regeneration patterns in relationship to light heterogeneity in two old-growth beech–fir forest reserves in South East Europe. Forestry 80:431–443. https://doi.org/10.1093/forestry/cpm037
Sabatini FM, Burrascano S, Keeton WS et al (2018) Where are Europe’s last primary forests? Divers Distrib 24:1426–1439. https://doi.org/10.1111/ddi.12778
Šafar J (1954) Die Entwicklung des Tannenjungwuchses in den Plenterwäldern Kroatiens. Schweiz Z Forstwes 105:592–613
Savva Y, Oleksyn J, Reich P, Tjoelker M, Vaganov E, Modrzynski J (2006) Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees Struct Funct 20:735–746. https://doi.org/10.1007/s00468-006-0088-9
Schmied G, Hilmers T, Uhl E, Pretzsch H (2022) The past matters: previous management strategies modulate current growth and drought responses of Norway Spruce (Picea abies H. Karst.). Forests 13:243. https://doi.org/10.3390/f13020243
Schröter M, Härdtle W, von Oheimb G (2012) Crown plasticity and neighborhood interactions of European beech (Fagus sylvatica L.) in an old-growth forest. Eur J For Res 131:787–798. https://doi.org/10.1007/s10342-011-0552-y
Schütz JP (1975) Dynamique et conditions d’équilibre de peuplements jardinés sur les stations de la hêtraie à sapin. Schweiz Z Forstwes 126:637–671
Schütz JP (2001) Der Plenterwald und weitere Formen strukturierter und gemischter Wälder. Parey, Berlin
Schütz JP, Saniga M, Diaci J, Vrška T (2016) Comparing close-to-nature silviculture with processes in pristine forests: lessons from Central Europe. Ann For Sci 73:911–921. https://doi.org/10.1007/s13595-016-0579-9
Schweiger J, Sterba H (1997) A model describing natural regeneration recruitment of Norway spruce (Picea abies (L.) Karst.) in Austria. For Ecol Manage 97:107–118. https://doi.org/10.1016/S0378-1127(97)00092-3
Senécal JF, Doyon F, Messier C (2018) Tree death not resulting in gap creation: an investigation of canopy dynamics of northern temperate deciduous forests. Remote Sensing 10:121. https://doi.org/10.3390/rs10010121
Seymour RS, White AS, de Maynadier PG (2002) Natural disturbance regimes in northeastern North America—evaluating silvicultural systems using natural scales and frequencies. For Ecol Manage 155:357–367. https://doi.org/10.1016/S0378-1127(01)00572-2
Sidor CG, Popa I, Vlad R, Cherubini P (2015) Different tree-ring responses of Norway spruce to air temperature across an altitudinal gradient in the Eastern Carpathians (Romania). Trees Struct Funct 29:985–997. https://doi.org/10.1007/s00468-015-1178-3
Sillett SC, Van Pelt R, Carroll AL, Kramer RD, Ambrose AR, Trask DA (2015) How do tree structure and old age affect growth potential of California redwoods? Ecol Monogr 85:181–212. https://doi.org/10.1890/14-1016.1
Sillett SC, Van Pelt R, Koch GW, Ambrose AR, Carroll AL, Antoine ME, Mifsud BM (2010) Increasing wood production through old age in tall trees. For Ecol Manage 259:976–994. https://doi.org/10.1016/j.foreco.2009.12.003
Sohn JA, Saha S, Bauhus J (2016) Potential of forest thinning to mitigate drought stress: a meta-analysis. For Ecol Manage 380:261–273. https://doi.org/10.1016/j.foreco.2016.07.046
Splechtna BE, Gratzer G, Black BA (2005) Disturbance history of a European old-growth mixed-species forest: a spatial dendro-ecological analysis. J Veg Sci 16:511–522. https://doi.org/10.1111/j.1654-1103.2005.tb02391.x
Stan AB, Daniels LD (2010) Growth releases of three shade-tolerant species following canopy gap formation in old-growth forests. J Veg Sci 21:74–87. https://doi.org/10.1111/j.1654-1103.2009.01120.x
Stan AB, Daniels LD (2014) Growth releases across a natural canopy gap-forest gradient in old-growth forests. For Ecol Manage 313:98–103. https://doi.org/10.1016/j.foreco.2013.11.004
Stancioiu PT, O’Hara KL (2006) Regeneration growth in different light environments of mixed species, multiaged, mountainous forests of Romania. Eur J For Res 125:151–162. https://doi.org/10.1007/s10342-005-0069-3
Stephenson NL, Das AJ, Condit R, Russo SE, Baker PJ, Beckman NG, Alvarez E (2014) Rate of tree carbon accumulation increases continuously with tree size. Nature 507:90–93. https://doi.org/10.1038/nature12914
Stiers E, Willim K, Seidel D, Ehbrecht M, Kabal M, Ammer Ch, Annighöfer P (2018) A quantitative comparison of the structural complexity of managed, lately unmanaged and primary European beech (Fagus sylvatica L.) forests. For Ecol Manage 430:357–365. https://doi.org/10.1016/j.foreco.2018.08.039
Szwagrzyk J, Szewczyk J (2001) Tree mortality and effects of release from competition in an old-growth Fagus-Abies-Picea stand. J Veg Sci 12:621–626. https://doi.org/10.2307/3236901
Thomas H (2013) Senescence, ageing and death of the whole plant. New Phytol 197:696–711. https://doi.org/10.1111/nph.12047
Vašíčková I, Šamonil P, Fuentes Ubilla AE, Král K, Daněk P, Adam D (2016) The true response of Fagus sylvatica L. to disturbances: a basis for the empirical inference of release criteria for temperate forests. For Ecol Manage 374:174–185. https://doi.org/10.1016/j.foreco.2016.04.055
Vencurik J, Kucbel S, Saniga M, Jaloviar P, Sedmáková D, Pittner J, Parobeková Z, Bosela M (2020) Growth dynamics of the Norway spruce and silver fir understory in continuous cover forestry. iForest 13:56–64. https://doi.org/10.3832/ifor3183-012
Vrška T, Adam D, Hort L, Janík KT (2009) European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) rotation in the Carpathians—a developmental cycle or a linear trend induced by man? For Ecol Manage 258:347–356. https://doi.org/10.1016/j.foreco.2009.03.007
Wenk G, Antanaitis V, Šmelko Š (1990) Waldertragslehre. VEB Deutscher Landwirtschaftsverlag, Berlin
Wertz B, Wilczyński S, Muter E (2014) Dendrochronologiczna ocena przyrostu grubości jodły pospolitej (Abies alba Mill.) w Polskich Karpatach. Studia i Materiały CEPL w Rogowie 40:88–98
Wilczyński S, Szymański N (2015) Sygnał klimatyczny w seriach przyrostów rocznych świerków z regla dolnego oraz górnego w Tatrach. Sylwan 159:1008–1017. https://doi.org/10.26202/sylwan.2015054.
Wilczyński S, Szymański N, Kołbut Ł (2015) Klimatyczne przyczyny krótkookresowych reakcji przyrostowych jodły pospolitej z pogórza oraz regla dolnego. Sylwan 159:372–380. https://doi.org/10.26202/sylwan.2017124./
Woods KD (2008) Living long by staying small: stem layering as an adaptive life-history trait in shade-tolerant tree seedlings. Can J for Res 38:480–487. https://doi.org/10.1139/X07-136
Wright EF, Canham CD, Coates KD (2000) Effects of suppression and release on sapling growth for 11 tree species of northern, interior British Columbia. Can J For Res 30:1571–1580. https://doi.org/10.1139/x00-089
Wypych A, Ustrnul Z, Schmatz DR (2018) Long-term variability of air temperature and precipitation conditions in the Polish Carpathians. J Met Sci 15:237–253. https://doi.org/10.1007/s11629-017-4374
Youngblood AP (1991) Radial growth after a shelterwood seed cut in a mature stand of white spruce in interior Alaska. Can J For Res 21:410–413. https://doi.org/10.1139/x91-052
Zapałowicz H (1880) Roślinność Babiej Góry pod względem geograficzno-botanicznym. Sprawozdanie Komisji Fizjograficznej PAU, Kraków 14:79–236
Acknowledgements
The authors wish to thank an anonymous reviewer for his/her helpful and pertinent comments on the original version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland (on contract No. 2014/15/N/NZ9/01143 to RJ).
Author information
Authors and Affiliations
Contributions
JP conceived the ideas and designed methodology, RJ collected the data; RJ analyzed the tree-ring data, JP performed analyses; JP wrote the manuscript; RJ and JP gave final approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter Annighöfer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paluch, J., Jastrzębski, R. Life histories of Abies alba and Picea abies growing in old-growth forests driven by natural gap-phase dynamics. Eur J Forest Res 142, 331–352 (2023). https://doi.org/10.1007/s10342-022-01525-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-022-01525-w