Abstract
Sustainable agricultural management practices aimed at improving soil health can alter the soil microbiome, which can influence plant health and defenses against insects. The western corn rootworm (WCR), Diabrotica virgifera virgifera LeConte, is a major belowground pest of corn. Pest management relies heavily on the planting of transgenic crops expressing Bacillus thuringiensis (Bt) toxins. In this study, we ask how corn-WCR interactions via the soil microbiome are affected by a conservation management system (extended corn-soybean-wheat rotation with cover crops under no-till) compared with a conventional management system (corn-soybean rotation under mulch tillage and no cover crops) when combined with transgenic Bt corn. To do this, we applied soil microbes from the conservation and conventional management systems to two corn lines, one producing Bt and one non-Bt. We then reared Bt-resistant and Bt-susceptible WCR on inoculated seedlings to examine plant and insect changes in fitness. We found that Bt was effective against susceptible larvae in both soil treatments. Bt-resistant larvae were ~ 20% smaller when reared in the presence of soil microbes from the conservation management system. Thus, control of Bt-resistant WCR may be improved in a conservation system without sacrificing Bt effectiveness in susceptible insects. Comparing the microbial communities using 16S rRNA sequencing revealed that management practices influenced the microbiomes associated with the soil and the plant rhizosphere, but not WCR. Our findings suggest value for growers in utilizing conservation management practices, such as no-till and cover crops, in agricultural systems through bottom-up changes to plant–insect interactions via the soil microbiome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Cropping practices influence the soil microbiome.
-
Western corn rootworm (WCR) is a major below-ground pest of corn.
-
WCR-corn interactions may be altered through changes in the soil microbiome.
-
Soil health practices alter rhizosphere microbiomes that correlate with reduced WCR fitness.
-
Cropping practices designed to boost soil health may improve below-ground pest management.
Introduction
A major goal of sustainable cropping practices is to maintain or improve soil health, defined as, “the continued capacity of a soil to function as a vital living ecosystem that sustains plants, animals, and humans” (e.g., Karlen et al. 2019). Soil health can be modified through management practices that change soil disturbance, residue and amendment inputs, soil cover, and crop diversity (Doran 2002; Veum et al. 2015, 2022). In turn, management systems integrating soil health conservation practices experience increased soil organic matter, reduced erosion, lower nutrient loss, higher pathogen suppression, and increased microbial abundance (Nunes et al. 2020a, 2020b; Veum et al. 2015, 2022; Hartwig and Ammon 2002; Kim et al. 2020).
Common practices aimed at improving soil health include planting cover crops, reducing tillage, and expanding crop rotations (USDA NASS 2017; Wallander et al. 2021). Instead of leaving a field fallow with bare soil over the winter, a grower can seed cover crops in the fall immediately before or after harvest of the cash crop. The cover crop will overwinter and be terminated the following spring. Although a corn yield drag has been observed under some conditions (Liedgens et al. 2004), cover crops can potentially increase cash crop production by improving several soil health metrics (Hartwig and Ammon 2002; Kim et al. 2020). Tillage operations are known to lead to dramatic losses in soil organic matter and soil health status, whereas reduced tillage or no-till systems can maintain or restore soil health (e.g., Nunes et al. 2020a, 2020b; Veum et al. 2015, 2022). Integrating cover crops into no-till systems can further boost surface soil carbon and water infiltration (Mitchell et al. 2017). Enhanced above-ground biodiversity, achieved through extended crop rotations, may contribute to improved crop productivity, nutrient cycling, pathogen suppression, and water dynamics (Tilman et al. 2014). In contrast, low diversity cropping systems, such as monoculture or two-crop systems, can reduce soil health and productivity (Schmer et al. 2020; Chahal et al. 2021). Growers are continuing to adopt practices increasing soil health; farmland acreage planted with cover crops totaled over 15 million in the USA in 2017, an increase of 50% from 2012 (USDA NASS 2017). Over 100 million acres of farmland practice no-till, and rates are 2–3 times higher in systems using cover crops (Wallander et al. 2021).
Soil management practices can also affect insect pest populations, an important concern for growers as insect pests reduce yields by 20% annually (Culliney 2014). The majority of studies investigating the effects of cover crops and no-till on insect pests focus on the benefits of promoting arthropod predator populations as a top-down control tactic (Bowers et al. 2021; Lundgren and Fergen 2010; Prasifka et al. 2006; Rivers et al. 2020), but these studies yield variable outcomes (Fox et al. 2016; Rowen and Tooker 2021). Cropping systems that incorporate multiple conservation practices, such as extended rotations with cover crops and reduced tillage, increase soil health by supporting diverse microbial communities and enhancing nutrient cycling and availability (Lehman et al. 2015). Yet considerably less work focuses on bottom-up pest suppression through changes in the soil microbiome. Moreover, studies investigating the effect of soil microbes on plant–insect interactions rarely examine the insect microbiome and instead focus on changes in the plant and its microbiome. Insects can use microbes to overcome plant defenses (Chu et al. 2013), supplement nutrition (Douglas 2009), and resist insecticides (Kikuchi et al. 2012). Oftentimes, these microbes are acquired from the environment (Kikuchi et al. 2007; van den Bosch and Welte 2017). Thus, changes in the plant or soil microbiome may disrupt insect microbiomes and impact insect pest management.
It is increasingly clear that soil microbiomes can improve plant health and alter plant defenses against pests (Chaparro et al. 2012; Pineda et al. 2010). Examples of microbe-induced pest resistance in cover crop systems exist (Blundell et al. 2020; Krey et al. 2020), but are often confounded with organic farming practices, of which cover crops are only one aspect of management. In reality, the vast majority of cover crop no-till acreage has been adopted on non-organic farms (USDA NASS 2017). Despite the growing interest in plant-soil-microbial interactions, the effects of management on belowground insects are rarely investigated (Leslie et al. 2017; Lundgren and Fergen 2010) even though root herbivory has significant impact on ecosystems (Hunter 2001). Therefore, it is necessary to investigate the effects of conservation management systems on belowground pest suppression via the soil microbiome in a more traditional agricultural setting.
One of the most damaging belowground crop pests in the USA is the western corn rootworm (WCR), Diabrotica virgifera virgifera LeConte. The immature larvae feed on corn roots causing severe damage that results in decreased plant nutrient uptake, decreased plant stability, and increased susceptibility to pathogens (Hou et al. 1997; Kahler et al. 1985; Kurtz et al. 2010; Riedell 1990; Spike and Tollefson 1991). The combined cost of damage and management of WCR accounts for an estimated $2 billion in 2010 (Wechsler and Smith 2018). WCR management relies heavily on transgenic corn that produces toxins derived from Bacillus thuringiensis (Bt). Consequently, the continuous planting of Bt corn has led to the evolution of resistance. All four commercially available transgenic Bt products (Cry3Bb1, mCry3a, eCry3.1Ab, and Gpp34/Tpp35) have seen failures in the field while the number of acres planted with Bt continues to increase (Gassmann 2021). Given the concurrent increase in cover crop utilization, it is important to understand how these two management strategies interact and how their interaction influences WCR management.
Current evidence suggests the WCR microbiome may be susceptible to management-driven disruption of microbial communities. It is likely that WCR are highly adapted to corn root microbes. There is overlap in corn root microbiomes and WCR microbiomes (Dematheis et al. 2012; Ludwick et al. 2019), but the extent to which larval WCR acquire microbes from the environment is not well understood (Ludwick et al. 2019). Larvae select for a relatively conserved bacterial community that is refined with age (Ludwick et al. 2019). Continuous selection on Bt alters the larval microbiome suggesting it may play a role in susceptibility and resistance to Bt (Paddock et al. 2021). As adults, WCR can use microbes to overcome plant defenses to exploit a new host plant (Chu et al. 2013), but their microbiome varies based on their geographical location (Paddock et al. 2022).
Here, we examined the impact of soil microbiomes from a long-term conservation management system [a corn (Zea mays L.)-soybean (Glycine max L. Merr)-wheat (Triticum aestivum) rotation under no-till with cereal rye (Secale cereale) cover crops] and a conventional management system (mulch till corn-soybean rotation without cover crops) on the microbiome of WCR feeding on two different corn lines (Bt and non-Bt). The aims of the study were to (1) characterize microbial communities from soil under differing management tactics, (2) observe differences in plant and insect microbiomes grown in the presence of those soil microbiomes, and (3) measure the impact of the microbiomes on plant and insect growth. Our hypothesis was that changes in soil microbiomes introduced through varying management practices would alter the corn root rhizosphere. These changes may then disrupt the microbiome of WCR larvae. Given the influence of microbiomes on host fitness, we sought to compare how these soil health management practices affect plant–insect interactions. We paired fitness assays with 16S rRNA gene sequencing to capture microbe-mediated changes to corn and WCR fitness. We hypothesized that rhizosphere community compositional changes would reduce WCR fitness in the presence of microbes from the conservation management system. Taken together, this study will inform future pest management decisions for growers integrating conservation management practices such as cover crops, no-till, and extended rotations into a traditional row crop setting.
Methods
Sample collection and preparation
The experimental site is located at the Goodwater Creek Experimental Watershed in Centralia, Missouri and managed by the USDA-ARS Cropping Systems and Water Quality Research Unit. The experimental plots were established in 1991 to study the productivity and environmental effects of conservation management practices. In 2012, the site was selected as one of the 10 initial ARS Long-Term Agroecosystem Research (LTAR) network sites and was named the Central Mississippi River Basin (LTAR-CMRB) site. Research conducted at this site has quantified the benefits of conservation management practices, including crop productivity, water quality, and soil health (Sadler et al. 2015; Veum et al. 2015). Ten management systems represent a continuum of variable crop rotation, tillage, and cover crop practices. Research plots at the site are 18 by 189 m (59 by 620 ft) running east–west and the experimental design is a randomized complete block with three replications (Supplementary Figure S1a). Soils at the site include Adco silt loam (fine, smectitic, mesic Vertic Albaqualfs) and Mexico silt loam (fine, smectitic, mesic Vertic Epiaqualfs). Cropping system treatments selected for this study included 1) a conservation management system in a corn-soybean-wheat rotation under no-till with cereal rye cover crops, and 2) a conventional management system in a corn-soybean rotation under mulch tillage with no cover crops (Supplementary Figure S1b). Further details of the management practices and history can be found in Supplementary Table S1.
In April 2021, soil cores were collected to 10 cm depth with a 3.18 cm diameter probe from the replicated conservation and conventional management system plots. Each system was in the corn phase of the crop rotation the year before. Crop residues were removed from the surface prior to sampling, and the soil probe was sterilized with 95% ethanol between locations. Five soil cores were collected and combined into a single composite sample at each of four locations within each plot (~ 150 ft apart) for a total of 12 soil samples per management system (Supplementary Figure S1c). Samples were transported back to the laboratory at the University of Missouri-Columbia in a cooler with ice packs where they were placed in a 4 °C refrigerator until further processing one week later.
Each soil sample was homogenized and passed through a steel sieve (1 cm) to remove any large debris. Sieved soil was homogenized by mixing, and 30 g were transferred to two separate 50-mL flip top tubes (Nalge Nunc International, Rochester, NY, USA). Three 1 mL soil samples were subsampled and stored at -80 °C for subsequent DNA extraction and sequencing. Soil slurries were prepared based on previously published methods (Walsh et al. 2021). To prepare microbial soil slurries, 37.5 mL of 1 × PBS (7.4 pH) was added to each tube, and the tubes were shaken on their side on a platform shaker (New Brunswick Scientific, Edison, New Jersey) at a speed of 40 rpm for 30 min. Soil debris was separated from the microbial layer by centrifugation at 600 g for 4 min (Walsh et al. 2021; Allegra 25R, Beckman Coulter, Brea, CA, USA). The supernatant was transferred to a clean 50-mL tube. Three 1 mL inoculum samples were also collected and stored at −80 °C for later DNA extraction and sequencing. Inoculum was used in bioassays the same day of preparation.
Bioassays
The experiment was designed as a 2 × 2 × 3 factorial design with 2 microbiome types (conservation and conventional), 2 corn types (Bt and non-Bt corn), and 3 rootworm infestation types (resistant, susceptible, and uninfested). Given the size of the experiment, a block was set up every other day over the course of one week for a total of four blocks matching the sampling location of the field. Thus, each sampling location within a replicated plot was treated as a block during the bioassay. Microbiome treatment and corn type were randomized within blocks, with one replicate per sample. Paired infested and uninfested treatments were used to calculate relative root damage. Each experimental unit was run in duplicate to allow for destructive fitness and microbiome sampling, resulting in a total of 288 experimental units.
Assays were conducted in 50-mL flip top tubes (Nalge Nunc International, Rochester, NY, USA). First, tubes were filled with 30 mL of soil mixture. The soil mixture of two parts top-soil and one part Promix (Premier Horticulture Inc., Quakertown, PA, USA) was double-autoclaved, allowed to cool, and passed through a sterile sieve (2 mm) to remove large soil particulates and rock before use in bioassays. The day before planting, corn seeds of both genotypes were surface sterilized by soaking in 5% bleach solution, triple-rinsed with sterile DI water and left to soak overnight to speed germination in the tubes. Each 50-mL tube was seeded with two kernels of corn and then overlaid with 1 mL of microbial inoculum per seed. The seeds were then covered with 10 mL of soil, watered with 5 mL of DI water, and left for 30 min to allow water to permeate the soil. An additional 5 mL of water was then added to each tube, and caps were closed for two days to retain moisture until seeds germinated inside a growth chamber (16:8 L:D). Five days after planting seeds, tubes were infested with six neonate larvae of either Bt-resistant (Frank et al. 2013; Geisert and Hibbard 2016; Paddock et al. 2021) or Bt-susceptible WCR (non-diapausing WCR; Crop Characteristics, Farmington, MN, USA) by transferring living larvae with a horse-hair paintbrush. Tubes were returned to growth chamber for the duration of the bioassay and watered as needed throughout (~ every 2 days).
Upon completion of the assay, tubes used for fitness measurements were emptied into Berlese funnels with a collection jar filled with water attached to the bottom to collect living larvae. Jars were collected after 24 h, and larvae were counted and placed in 1.5-mL tubes containing ethanol. Larvae were dried at 40 °C in an oven for one week. Plant fitness data were collected as follows. Aboveground tissue on corn seedlings was removed at the base of the mesocotyl. The remaining tissue was collected and rehydrated for 24 h, washed to remove excess soil, and weighed. Roots were then allowed to dry in oven at 40 °C for one week after which dry weights were recorded.
For microbiome sample collection, contents of the 50-mL tubes were emptied into a sterile metal container. Resistant larvae found moving were collected with a horse-hair paintbrush, surfaced sterilized with 70% ethanol, and transferred to sterile garnet beaded tubes (4 larvae max per tube). Tubes were frozen at −80 °C. Roots were lightly disturbed to separate soil from the rhizosphere (~ 1 mm surrounding roots). The roots and rhizosphere were transferred to sterile 50-mL flip top tube filled with 30 mL of 1 × PBS. Tubes were fastened, shaken by hand, and then allowed to sit for 30 min to settle the rhizosphere soil before debris was removed. Rhizosphere samples in tubes were spun down at 3000 g for 15 min in a high-speed centrifuge (Allegra 25R, Beckman Coulter, Brea, CA, USA) to pellet the microbial layer. Supernatant layer was pipetted off leaving the rhizosphere sample undisturbed. Rhizosphere subsamples of equal weight (250 mg) were transferred to sterile 1.5-mL beaded garnet tubes in triplicate and immediately frozen at −80 °C.
DNA extraction and 16S rRNA gene amplification
Bacterial DNA was extracted from the original soil samples, microbial inoculum, plant rhizospheres, and living, Bt-resistant insects using PowerFecal Pro DNA Isolation kits (Qiagen, catalogue No. 51804) in accordance with manufacturer protocols (https://www.qiagen.com/us/resources/resourcedetail?id=8896817a-253f-4952-b845-0aab796813ce&lang=en). The total sample list can be found with the metadata on FigShare at doi: 10.6084/m9.figshare.22229527. DNA concentration was measured using a Qubit 2.0 fluorometer (Thermo Fisher Scientific) to ensure equal concentrations of 3.51 ng/µL. Extracted DNA was stored at −80 °C until downstream processing began. 16S amplicon libraries were constructed and sequenced at the MU DNA Core in Columbia, Missouri. The V4 hypervariable region of the 16S rRNA gene was amplified using single indexed universal primers (515F/806R; Caporaso et al. 2012) with Illumina standard adapter sequences. Forward and reverse, dual indexed primers were used in all reactions. PCR steps were as follows: 98C(3:00) + [98C(0:15) + 50C(0:30) + 72C(0:30)] for 25 cycles. The resulting amplicons were pooled before sequencing on Illumina MiSeq 2 × 250 bp platform.
16S rRNA sequence assembly
16S rRNA sequence processing was conducted using Qiime2 v2022.8 (Bolyen et al. 2019). Paired-end reads were demultiplexed prior to trimming primer sequences with Cutadapt (Martin 2011). An error rate of 0.1 was allowed in the primer sequences, and any untrimmed reads were discarded. Low-quality filtering and denoising were performed with DADA2 (Callahan et al. 2016). Reads were trimmed when the lower 25th quartile range at a given base pair fell below a quality score of 30. Chimeras were detected using the “consensus” method and removed. Remaining sequences were filtered to retain those with lengths between 240 and 255. Taxonomy was assigned to amplicon sequence variants (ASVs) using the Silva.v132 database with the sklearn classifier in Qiime2 (Quast et al. 2012; Bokulich et al. 2018). ASVs were compiled into biom tables for downstream analysis in RStudio 4.0.4. Any ASV matching chloroplast, mitochondria, archaea, Wolbachia, or “uncharacterized” at the phylum level were filtered using phyloseq:filter_taxa (McMurdie and Holmes 2013). For global comparisons across sample types, data were rarefied to even depth of 1300 reads.
Data analysis
All data analyses were conducted in RStudio 4.0.4. We first tested the effect that soil microbes from different management systems had on plant–insect interactions between Bt-resistant and -susceptible insects feeding on Bt and non-Bt corn. Larval fitness was estimated by measuring larval survival and average larval dry weight of surviving insects. All surviving insects collected from an experimental unit were weighed together and divided by the number of larvae to obtain the average weight per larvae. Residuals of dependent variables were checked for normality of variance using Levene’s test and normality of distributions using Shapiro–Wilk test prior to evaluation in linear mixed effects models. Average larval dry weight data did not satisfy the assumptions of normality of residuals and were square-root transformed. The main effects of microbiome treatment, corn line, and colony and their interactions were examined using a linear mixed effects model with block, block × corn line, and block × colony interactions as the random effects (dry weight ~ microbiome treatment × corn line × colony + block + block × corn line + block × colony). Post hoc comparisons of estimated marginal means were conducted on significant interactions using emmeans in the emmeans package based on a priori predictions (Russell 2022). Mortality was modeled using a generalized mixed effect model following a Poisson distribution with microbiome treatment, corn line, and colony and their interactions as main effects and a random slope of block and random intercept of corn line and colony. Type III sum of squares were compared using Anova in the car package (Fox and Weisberg 2019), and post hoc tests on estimated marginal means were conducted on significant interactions using emmeans based on a priori predictions. To evaluate changes in plant growth, we compared the effect of soil microbes on root dry weight of uninfested control plants. Data were analyzed using a linear mixed effects model with treatment x corn line interaction as the main effects and block and block x corn line interaction as the random effects in a single model. Post hoc comparisons of estimated marginal means were conducted on significant effects. Log response ratios for corn root dry weight were calculated by taking the natural log of the proportion of root dry weight of WCR infested plants to the paired uninfested control plant. Log response ratios were analyzed using a linear mixed effects model with treatment × corn line × colony interaction as the main effects and block, block × colony, and block × corn line interaction as the random effects. Post hoc comparisons of estimated marginal means were conducted on significant effects.
Next, we examined the overall effect of soil management system on the microbiome alpha and beta diversity of each sample type (soil, inoculum, rhizosphere, insect). Alpha diversity was calculated using estimate_richness in the phyloseq package. Estimates of richness and Inverse Simpson’s D were evaluated using a linear mixed effects model with management tactic × sample type interaction with block as the random effect. Residuals of dependent variables were checked for normality of distributions using Shapiro–Wilk test prior to evaluation in linear mixed effects models. Post hoc comparisons of estimated marginal means were conducted on significant interactions using emmeans in the emmeans package. We analyzed beta diversity using a single PERMANOVA model with field block as the random effect and management tactic x sample type interaction with adonis2 in the vegan package (Oksanen et al. 2019). A significant interaction was followed by pairwise comparisons using pairwise.adonis2 treating each treatment × sample type combination as an independent factor (Martinez Arbizu 2020). Variance within a community was estimated using betadisper and compared using a permutational test of multivariate homogeneity of group dispersion treating each treatment × sample type combination as an independent factor in the vegan package. Pairwise differences were compared using TukeyHSD.
We followed up the global tests with specific models investigating the differences between management tactics on rhizospheres of Bt and non-Bt corn fed upon by different insect strains (Bt-resistant, Bt-susceptible, and uninfested) using unrarefied data given the low variation in sampling depth between samples. We compared differences in overall community composition using Bray–Curtis and Jaccard distances in a permutational analysis of variance (PERMANOVA) with treatment × corn line × colony interaction as the main effects and field block as the random effect (specified through permutation). Significant interactions were tested using individual models across levels of an effect. NMDS ordinations based on Bray–Curtis distances were generated using ordinate in the phyloseq package. Alpha diversity metrics were calculated using estimate_richness in the phyloseq package. Estimates of richness and Inverse Simpson’s D were evaluated using a linear mixed effects model with treatment, corn line, colony, and their interaction as fixed effects and block, block × corn line, and block × colony interaction as random effects. Dependent variables were checked for normality of distributions using Shapiro–Wilk test prior to evaluation in linear mixed effects models. Post hoc comparisons of estimated marginal means were conducted on significant interactions using emmeans in the emmeans package.
We examined bacterial taxa that were differentially abundant between treatments using analysis of compositions of microbiomes with bias correction (ANCOM-BC; Lin and Peddada 2020). We made comparisons between those samples that had statistically different microbial communities based on PERMANOVA results, and each corn line was analyzed separately to account for differences between the lines. Analyses were conducted at two different taxonomic ranks, family and ASV level. Resulting taxa that were found to be significantly differentially abundant between treatments were filtered to select the most abundant 125 ASVs across samples. The top 125 were used to visualize the log10 percent abundance across sample types using a heatmap. Recruitment from the soil microbiome by plants was estimated as the proportion of reads observed in the rhizosphere of each corn line in each soil microbiome treatment consistent with the field replication. Proportions were analyzed using beta regression with the treatment corn line interaction using betareg (Cribari-Neto and Zeileis 2010).
Results
To assess the impact of microbial communities on corn-WCR interactions, we first examined dry weight and larval survival of Bt-resistant and -susceptible WCR feeding on Bt and non-Bt corn inoculated with soil microbiomes from contrasting management systems. There were differences in how Bt-resistant and -susceptible WCR responded to the soil microbiome treatment (treatment × colony interaction: F1,96 = 6.643, p = 0.0123). Susceptible WCR dry weight was not affected by the soil microbiome treatment (Fig. 1a). However, Bt-resistant WCR dry weight was significantly reduced when reared on corn treated with soil microbes from the conservation management system (Fig. 1b). Bt was equally effective in both soil microbiome treatments at controlling susceptible insects, both in terms of mortality and dry weight (weight; corn line × colony interaction: F1,96 = 28.31, p < 0.001; mortality; corn line x colony interaction: F1,96 = 18.51, p < 0.001, df = 1,96; Fig. 1a). We also examined the impact of the soil microbiome on plant fitness by comparing dry weight of corn roots. Overall, there was a marginally significant increase in root weight when inoculated with the conservation management soil microbiome (Fig. 2a; p = 0.058). In addition, we found non-Bt roots to be heavier than Bt roots (Fig. 2a; p < 0.001); however, these were not true isolines because these were not available. Whether roots were fed upon by Bt-resistant and Bt-susceptible insects did not influence root biomass. We observed only a main effect of soil microbiome treatment on the log response ratio of biomass of fed upon to unfed upon roots. There was a significantly greater difference between fed upon and unfed upon roots when reared in the presence of the conservation management microbiome (Fig. 2b, F1, 96 = 4.661, p = 0.034).
Average larval dry weight of a Bt-susceptible and b Bt-resistant western corn rootworm (Diabrotica virgifera virgifera LeConte) when reared for five days on either Bt or non-Bt corn treated with soil microbiomes from conservation and conventional management systems. Each bar represents the average for each treatment across blocks. Error bars represent the standard error for each treatment combination across blocks. Pairwise differences are based on estimated marginal means from linear models where significance was considered for factors with p < 0.05. Single asterisk denotes p < 0.05; three asterisks denote p < 0.001
a Dry root weight of corn lines (Bt or non-Bt) grown for 10 days after inoculation with soil microbiomes from either conservation or conventional management systems. b Log response ratio of dry root weight between WCR infested and uninfested corn plants after 5 days. Sample means are presented as a bold point with accompanying standard error bars. Pairwise differences are based on estimated marginal means from linear models where significance was considered for factors with p < 0.05
We then sought to examine how bacterial communities of soil, plant, and insect were impacted by management practices. Overall, we found evidence that the long-term use of conservation management significantly alters the soil microbiome composition. However, this effect was variable across rhizosphere and WCR samples. Rhizosphere samples were significantly influenced by the soil microbiome applied to the seedlings, both in terms of community richness, diversity, and composition (Supplementary Figure S2a–c). Rhizosphere richness was increased when corn seedlings were grown in the presence of the conservation management soil microbiome compared to the conventionally managed soil microbiome (pairwise: t = 5.176, p < 0.001), despite there being no difference in the richness of the original soil microbiome. WCR larval microbiome composition did not vary based on the soil microbiome applied to their host plant (Supplementary Figure S2a). Inverse Simpson’s D, or the effective number of species, followed a similar pattern with the highest diversity in soil, followed by rhizosphere, and insect samples. Insects harbored the communities with the lowest diversity (Supplementary Figure S1c). Beta diversity analyses revealed a significant interaction between sample type and microbiome treatment (type × treatment: F3,228 = 2.54, p < 0.001, R2 = 0.0214). Pairwise comparisons between each combination resulted in significant differences in centroid location for all pairs except for WCR larval microbiomes reared in different soil microbiomes (Supplementary Table S2).
To specifically investigate whether changes in WCR fitness could be explained by rhizosphere communities, we analyzed data from rhizosphere communities of the two different corn lines, Bt and non-Bt, infested with either Bt-resistant WCR, Bt-susceptible WCR, or uninfested. We found a significant treatment by corn line interaction for richness and community composition. Diversity estimated by Inverse Simpson’s D was not different across any of the conditions. For rhizosphere richness, the interaction was due to an observed increase in community richness in Bt corn rhizospheres grown in the presence of cover crop soil microbes, but no difference in non-Bt corn rhizosphere richness (Fig. 3c; pairwise comparisons: Bt: p < 0.001; non-Bt: p = 0.18). These differences in corn line may be driven by the increased variance in richness of non-Bt corn rhizospheres (Levene’s test: corn line, p = 0.03). Soil microbes from different management systems significantly altered community composition based on Bray–Curtis and Jaccard distances in both Bt and non-Bt corn rhizospheres but by different magnitudes (B-C treatment × corn line: p = 0.008; Jaccard treatment × corn line: p = 0.005). Bt-corn rhizosphere communities varied more substantially between treatments compared to non-Bt corn rhizospheres (Supplementary Table S3). When comparing corn lines within each soil microbiome treatment, we only found differences between corn lines when reared in the conventionally managed soil microbiome (Supplementary Table S3). We did not detect differences in beta-dispersion between those groups. In addition, we found no evidence feeding by either insect strain altered rhizosphere communities compared to unfed upon corn rhizospheres.
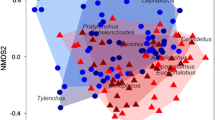
a Relative abundance of different classes of bacteria found in different sample types. Each bar represents the combined abundances of all samples within the specified treatment group rarefied to 1300 reads. Conservation and conventional management systems are distinguished by colors, corn lines are distinguished by stripes, and insect colonies are distinguished by labels. b Non-metric multidimensional scaling of 10-day old Bt or non-Bt rhizosphere bacterial communities inoculated with conservation or conventional management system soil microbiomes with unrarefied data. c Comparison of number of observed species present in the rhizosphere of Bt and non-Bt corn inoculated with bacterial communities from conservation or conventional management system soils. Three asterisks denote p < 0.001
Soil microbiomes from the two management systems were composed of similar classes of bacteria but in different relative abundance (Fig. 3a). The conservation soil microbiome contributed a higher proportion of taxa from their bacterial communities to the corn rhizosphere (p = 0.0144). While the proportion was relatively small when accounting for only presence or absence, these taxa combined to account for around ~ 40% of the rhizosphere communities. Differentially abundant taxa between soil microbiome treatments in the rhizosphere of each corn line were concentrated across nine classes of bacteria (Fig. 4b). A total of 118 ASVs overlapped in enrichment in the rhizosphere of both corn lines (Fig. 4a). Some genera were found enriched in only one treatment, whereas other genera were represented in both management systems. For insects, we found an average of 127 ASVs also observed in the soil and rhizosphere samples, which represents an average of 14.64% of the total taxa observed across samples.
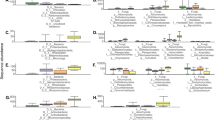
a Heatmap of the top 125 most abundant ASV found to be significantly differentially abundant between treatments in both corn lines using separate ANCOM-BC with significance at p < .05. Each column represents an individual rhizosphere sample from the corresponding corn line and microbiome treatment, and each row represents an individual ASV grouped by class. The log10 relative abundance was calculated as a proportion of the ASV in the individual sample divided by the total sum of all reads in that sample. b Differentially abundant genera between Bt- corn rhizospheres inoculated with conservation or conventional management system soil microbiomes. Significant differences in abundance between treatments were calculated using an analysis of compositions of microbiomes with bias correction (ANCOM-BC) with significance at p < 0.05
Discussion
Sustainable cropping practices can boost crop yield while maintaining broader ecosystem functioning. Practices promoting soil health such as extended rotations, cover crops, and reduced tillage, can increase plant health through changes in the soil microbiome (Lehman et al. 2015). Here, we document the soil microbiome of a conservation management system reduces Bt-resistant western corn rootworm fitness potentially through an increase in rhizosphere microbial richness. Changes in WCR fitness are likely plant-mediated effects as we detected no difference in the microbiome of WCR feeding on corn roots in conservation soil microbiomes compared to conventional soil microbiomes. Root weight for Bt plants was higher when grown in association with the conservation soil microbiome, further highlighting a correlation between rhizosphere richness and plant health. In addition, susceptibility to Bt was still high in Bt-susceptible insects regardless of the soil microbiome. Growers may achieve improved control of Bt-resistant WCR when using Bt corn in their conservation management system without sacrificing Bt effectiveness in non-resistant insects.
Conservation management practices can impact pest populations. One study that investigated the effects of cover crops on WCR found that WCR survival and plant damage decreased in a cover crop system compared to a traditional one (Lundgren and Fergen 2010). The authors concluded that increased predator abundance in cover crop fields was responsible for reduced 3rd instar WCR abundance and plant damage. Interestingly, they also found that 2nd instar larvae were larger in traditionally managed fields, suggesting fitness decreased in cover crop fields, possibly to due to bottom-up effects via the soil microbiome. The conservation management practices significantly altered soil microbiomes in our study, and these differences translated to distinct rhizosphere communities. Plants recruit mutualistic microbes with which they trade photosynthetic carbon for increased soil resource uptake and/or increased stress tolerance (Hu et al. 2018). In our study, conservation management soils contained a greater number of taxa that formed association with the corn rhizosphere. This may be a result of soil management that minimizes disturbances and the time spent bare, which allows the build-up of microbial communities that are able to form associations with plants. The rhizosphere community provides plants with important cues to stimulate defense against pathogens and pests. These changes are often associated with induced systemic resistance (ISR), a primed defense state that allows a plant to respond more quickly and/or strongly to attacks by pathogens and herbivores (Pieterse et al. 2014). Native corn root defenses against WCR can limit root damage and WCR fitness (Brkić et al. 2020; El Khishen et al. 2009; Hibbard et al. 2007), but how their expression is altered by microbial communities is relatively unknown. Our work builds on the understanding that conservation management practices can build soil health while influencing plant health and pest suppression.
Surprisingly, we found evidence that WCR herbivory on corn roots was greater under conservation management. The difference in root weight between infested and uninfested corn roots was smaller for plants inoculated with the conventional soil microbiome. The age and length of our experiment may skew the amount of damage observed. WCR often clip roots when feeding (Kahler et al. 1985), especially in new nodes of roots beginning to emerge from the stalk, which may compound the loss of root mass over time. This may explain the high level of variation within and between treatments in our study. It may be that the reduced ratio in conservation management systems is a result of altered defense or nutrition in the corn roots. Insects can compensate for poor diets by increasing feeding rates (Lavoie and Oberhauser 2004). One plant compound that negatively impacts insect nutrition is lignin (Campbell and Sederoff 1996). Root lignin content can be altered by both soil microbial communities and WCR feeding (Bennett et al. 2015; Xue et al. 2012). Corn roots may be able to boost their lignin content in the presence of certain microbes when fed upon by WCR and reduce WCR fitness, a trade-off with overall growth of the plant. The reduced WCR growth could increase mortality over time (Benrey and Denno 1997). Our study only investigated the impact on 1st instar WCR and thus did not capture the impact of the soil microbiome on later developmental stages of WCR.
We found no difference in the microbiome of the WCR when reared in different soil microbiomes or on different corn plants. Our findings corroborate previous work with WCR larval microbiome and the soil microbiome (Dematheis et al. 2012; Ludwick et al. 2019); WCR larvae exhibit tight control over their microbiome. Regulation of the larval microbiome may be especially beneficial when feeding on plants that display strong defense against WCR (Chu et al. 2013; Mason et al. 2019; Paddock et al. 2021). While we didn’t observe whole community shifts, there may still be individual bacterial taxa altering WCR fitness. For example, other studies found feeding on corn infected with the fungus Fusarium verticillioides (Saccardo) Nirenberg decreased larval fitness (Kurtz et al. 2010), and several Serratia strains identified on roots fed upon by WCR were also observed in diseased adults (Prischmann et al. 2008). In this study, we also did not find evidence of changes to the rhizosphere microbiome as a whole when fed upon by WCR. However, our experiment was conducted with neonate larvae feeding over a period of five days, which may not induce enough damage to stimulate whole community remodeling.
Community composition of conservation soil microbiomes can be influenced through edaphic factors introduced by the practice (Hartmann and Six 2023), the cover crop plant species used (Nevins et al. 2018), and the timing of cover crop termination (Nevins et al. 2018). By using a replicated, long-term experimental site where climate and edaphic characteristics are controlled, we account for the edaphic factors that exist following microbial isolation. However, we do not know how our results would change under varying cover crop management practices, such as cover crop species/mix, seeding rate and method, or termination timing and method. The increased community richness observed in rhizosphere samples may attenuate community composition variation from these factors. In other words, rhizosphere richness reduces WCR weight. Fungi can also impact plant health in similar ways to bacteria and have been shown to alter bacterial communities themselves. We have not characterized fungal communities, but they likely are involved in the plant–insect interactions observed here. Genetic differences in corn lines can manifest as distinct rhizosphere communities (Meier et al. 2022; Walters et al. 2018). The interplay between plant genetics and rhizobiomes can results in distinct phenotypes in corn (Wagner et al. 2021). The two corn lines used in this study are genetically distinct in addition to the presence of transgenic Bt toxin production. The impact Bt toxins have on the microbial ecosystem in largely unknown. How cover crops may affect yield in the field is highly variable and dependent on the system (Marcillo and Miguez 2017).
Modern agriculture, typified by monoculture row crops or simple two-crop rotations managed with heavy chemical inputs, fallow periods, and high levels of soil disturbance, negatively impacts microbial communities (French et al. 2021). Ecological intensification of agriculture, which utilizes management practices that minimize negative environmental impacts while maintaining yield, offers a more sustainable approach (Bommarco et al. 2013). However, our understanding of how sustainable conservation management practices affect microbiomes and, vice versa, how microbiomes affect management, is lacking (French et al. 2021). Unified microbiome research across climates, soils, and crops may provide insight into the sustainable management of pests.
Data availability
Sequence data have been deposited on NCBI SRA under Bioproject accession number PRJNA929989. Raw data and accompanying metadata can be found at FigShare at doi: 10.6084/m9.figshare.22229527 and 10.6084/m9.figshare.22229563. Code used for statistical analyses can be found at FigShare at doi:10.6084/m9.figshare.22229638.
Change history
15 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10340-024-01751-8
References
Bennett AE, Grussu D, Kam J, Caul S, Halpin C (2015) Plant lignin content altered by soil microbial community. New Phytol 206:166–174. https://doi.org/10.1111/nph.13171
Benrey B, Denno RF (1997) The slow-growth–high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78:987. https://doi.org/10.2307/2265852
Blundell R, Schmidt JE, Igwe A, Cheung AL, Vannette RL, Gaudin ACM, Casteel CL (2020) Organic management promotes natural pest control through altered plant resistance to insects. Nat Plants 6:483–491. https://doi.org/10.1038/s41477-020-0656-9
Bokulich NA, Kaehler BD, Rideout JR et al (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:1–17. https://doi.org/10.1186/s40168-018-0470-z
Bolyen E, Rideout JR, Dillon MR et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Bommarco R, Kleijn D, Potts SG (2013) Ecological intensification: harnessing ecosystem services for food security. Trends Ecol Evol 28:230–238. https://doi.org/10.1016/j.tree.2012.10.012
Bowers C, Toews MD, Schmidt JM (2021) Winter cover crops shape early-season predator communities and trophic interactions. Ecosphere 12:e03635. https://doi.org/10.1002/ecs2.3635
Brkić A, Šimić D, Jambrović A, Zdunić Z, Ledenĉan T, Raspudić E, Brmeţ M, Brkić J, Mazur M, Galić V (2020) QTL analysis of western corn rootworm resistance traits in maize ibm population grown in continuous maize. Genetika 55:137–148. https://doi.org/10.2298/GENSR2001137B
Campbell MM, Sederoff RR (1996) Variation in lignin content and composition (mechanisms of control and implications for the genetic improvement of plants). Plant Physiol 110:3–13. https://doi.org/10.1104/pp.110.1.3
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N et al (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Chahal I, Hooker DC, Deen B, Janovicek K, Van Eerd LL (2021) Long-term effects of crop rotation, tillage, and fertilizer nitrogen on soil health indicators and crop productivity in a temperate climate. Soil till Res 213:105121. https://doi.org/10.1016/j.still.2021.105121
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499. https://doi.org/10.1007/s00374-012-0691-4
Chu CC, Spencer JL, Curzi MJ, Zavala JA, Seufferheld MJ (2013) Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc Natl Acad Sci USA 110:11917–11922. https://doi.org/10.1073/pnas.1301886110
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw 34:1–24. https://doi.org/10.18637/jss.v034.i02
Culliney TW (2014) Crop losses to arthropods. In: Pimentel D, Peshin R (eds) Integrated pest management. Springer, Dordrecht, pp 201–225. https://doi.org/10.1007/978-94-007-7796-5_8
Dematheis F, Kurtz B, Vidal S, Smalla K (2012) Microbial communities associated with the larval gut and eggs of the western corn rootworm. PLoS ONE 7:e44685. https://doi.org/10.1371/journal.pone.0044685
Doran JW (2002) Soil health and global sustainability: translating science into practice. Agric Ecosyst Environ 88: 119–127. https://doi.org/10.1016/S0167-8809(01)00246-8
Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47. https://doi.org/10.1111/j.1365-2435.2008.01442.x
El Khishen AA, Bohn MO, Voldseth PDA, Dashiell KE, French BW, Hibbard BE (2009) Native resistance to western corn rootworm (Coleoptera: Chrysomelidae) larval feeding: characterization and mechanisms. J Econ Entomol 102:2350–2359. https://doi.org/10.1603/029.102.0642
Fox AF, Kim TN, Bahlai CA, Woltz JM, Gratton C, Landis DA (2016) Cover crops have neutral effects on predator communities and biological control services in annual cellulosic bioenergy cropping systems. Agric Ecosyst Environ 232:101–109. https://doi.org/10.1016/j.agee.2016.07.003
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd Edition, Thousand Oaks CA, Sage, https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Frank DL, Zukoff A, Barry J, Higdon ML, Hibbard BE (2013) Development of resistance to eCry3.1Ab-expressing transgenic maize in a laboratory selected population of western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 106:2506–2513. https://doi.org/10.1603/EC13148
French E, Kaplan I, Iyer-Pascuzzi A, Nakatsu CH, Enders L (2021) Emerging strategies for precision microbiome management in diverse agroecosystems. Nat Plants 7:256–267. https://doi.org/10.1038/s41477-020-00830-9
Gassmann AJ (2021) Resistance to Bt maize by western corn rootworm: effects of pest biology, the pest–crop interaction and the agricultural landscape on resistance. InSects 12:136. https://doi.org/10.3390/INSECTS12020136
Geisert RW, Hibbard BE (2016) Evaluation of potential fitness costs associated with eCry3.1Ab resistance in Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J Econ Entomol 109:1853–1858. https://doi.org/10.1093/jee/tow095
Hartmann M, Six J (2023) Soil structure and microbiome functions in agroecosystems. Nat Rev Earth Environ 4:4–18. https://doi.org/10.1038/s43017-022-00366-w
Hartwig NL, Ammon HU (2002) Cover crops and living mulches. Weed Sci 50:688–699. https://doi.org/10.1614/0043-1745(2002)050[0688:aiacca]2.0.co;2
Hibbard BE, Willmot DB, Garcia FSA, Darrah LL (2007) Registration of the maize germplasm CRW3(S1)C6 with resistance to western corn rootworm. J Plant Regist 1:151–152. https://doi.org/10.3198/jpr2006.12.0774crg
Hou X, Meinke LJ, Arkebauer TJ (1997) Soil moisture and larval western corn rootworm injury: influence on gas exchange parameters in corn. J Agron 89:709–717. https://doi.org/10.2134/agronj1997.00021962008900050001x
Hu L, Robert CA, Cadot S et al (2018) Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 9:2738. https://doi.org/10.1038/s41467-018-05122-7
Hunter MD (2001) Out of sight, out of mind: the impacts of root-feeding insects in natural and managed systems. Agric for Entomol 3:3–9. https://doi.org/10.1046/j.1461-9563.2001.00083.x
Kahler AL, Olness AE, Sutter GR, Dybing CD, Devine OJ (1985) Root damage by western corn rootworm and nutrient content in maize. J Agron 77:769–774. https://doi.org/10.2134/agronj1985.00021962007700050023x
Karlen DL, Veum KS, Sudduth KA, Obrycki JF, Nunes MR (2019) Soil health assessment: past accomplishments, current activities, and future opportunities. Soil till Res 195:104365. https://doi.org/10.1016/j.still.2019.104365
Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Fukatsu T (2012) Symbiont-mediated insecticide resistance. Proc Natl Acad Sci 109:8618–8622. https://doi.org/10.1073/pnas.1200231109
Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73:4308–4316. https://doi.org/10.1128/AEM.00067-07
Kim N, Zabaloy MC, Guan K, Villamil MB (2020) Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol Biochem 142:107701. https://doi.org/10.1016/j.soilbio.2019.107701
Krey KL, Nabity PD, Blubaugh CK, Fu Z, Van Leuven JT, Reganold JP, Berim A, Gang DR, Jensen AS, Snyder WE (2020) Organic farming sharpens plant defenses in the field. Front Sustain Food Syst 4:97. https://doi.org/10.3389/fsufs.2020.00097
Kurtz B, Karlovsky P, Vidal S (2010) Interaction between western corn rootworm (Coleoptera: Chrysomelidae) larvae and root-infecting Fusarium verticillioides. Environ Entomol 39:1532–1538. https://doi.org/10.1603/en10025
Lavoie B, Oberhauser KS (2004) Compensatory feeding in Danaus plexippus (Lepidoptera: Nymphalidae) in response to wariation in host plant quality. Environ Entomol 33:1062–1069. https://doi.org/10.1603/0046-225X-33.4.1062
Lin H, Peddada SD (2020) Analysis of compositions of microbiomes with bias correction. Nat Commun 11:1–11. https://doi.org/10.1038/s41467-020-17041-7
Lehman RM, Acosta-Martinez V, Buyer JS et al (2015) Soil biology for resilient, healthy soil. J Soil Water Conserv 70:12–18. https://doi.org/10.2489/jswc.70.1.12A
Leslie AW, Wang K-H, Meyer SLF, Marahatt S, Hooks CRR (2017) Influence of cover crops on arthropods, free-living nematodes, and yield in a succeeding no-till soybean crop. Appl Soil Ecol 117–118:21–31. https://doi.org/10.1016/j.apsoil.2017.04.003
Liedgens M, Frossard E, Richner W (2004) Interactions of maize and Italian ryegrass in a living mulch system: (2) nitrogen and water dynamics. Plant Soil 259:243–258. https://doi.org/10.1023/B:PLSO.0000020965.94974.21
Ludwick DC, Ericsson AC, Meihls LN, Gregory MLJ, Finke DL, Coudron TA, Hibbard BE, Shelby KS (2019) Survey of bacteria associated with western corn rootworm life stages reveals no difference between insects reared in different soils. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-51870-x
Lundgren JG, Fergen JK (2010) The effects of a winter cover crop on Diabrotica virgifera (Coleoptera: Chrysomelidae) populations and beneficial arthropod communities in no-till maize. Environ Entomol 39:1816–1828. https://doi.org/10.1603/EN10041
Marcillo GS, Miguez FE (2017) Corn yield response to winter cover crops: an updated meta-analysis. J Soil Water Conserv 72:226–239. https://doi.org/10.2489/jswc.72.3.226
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10–12. https://doi.org/10.14806/ej.17.1.200
Martinez Arbizu P (2020) pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4
Mason CJ, Ray S, Shikano I, Peiffer M, Jones AG, Luthe DS, Hoover K, Felton GW (2019) Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome. Proc Nat Acad Sci 116:15991–15996. https://doi.org/10.1073/PNAS.1908748116
McMurdie PJ, Holmes S (2013) Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217. https://doi.org/10.1371/journal.pone.0061217
Meier MA, Xu G, Lopez-Guerrero MG, Li G, Smith C, Sigmon B, Herr JR, Alfano JR, Ge Y, Schnable JC, Yang J (2022) Association analyses of host genetics, root-colonizing microbes, and plant phenotypes under different nitrogen conditions in maize. Elife 11:e75790. https://doi.org/10.7554/eLife.75790
Mitchell JP, Shrestha A, Mathesius K, Scow KM, Southard RJ, Haney RL, Schmidt R, Munk DS, Horwath WR (2017) Cover cropping and no-tillage improve soil health in an arid irrigated cropping system in California’s San Joaquin Valley, USA. Soil till Res 165:325–335. https://doi.org/10.1016/j.still.2016.09.001
Nevins CJ, Nakatsu C, Armstrong S (2018) Characterization of microbial community response to cover crop residue decomposition. Soil Biol Biochem 127:39–49. https://doi.org/10.1016/j.soilbio.2018.09.015
Nunes MR, Karlen DL, Veum KS, Moorman TB, Cambardella CA (2020a) Biological soil health indicators respond to tillage intensity: a US meta-analysis. Geoderma 369:114335. https://doi.org/10.1016/j.geoderma.2020.114335
Nunes MR, van Es HM, Veum KS, Amsili J, Karlen D (2020) Anthropogenic and inherent effects on soil organic carbon across the U.S. Sustainability 12:5695. https://doi.org/10.3390/su12145695
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Wagner H (2019) Vegan: community ecology package. R package version 2.5–2. Cran R
Paddock KJ, Finke DL, Kim KS, Sappington TW, Hibbard BE (2022) Patterns of microbiome composition vary across spatial scales in a specialist insect. Front Microbiol 13:898744. https://doi.org/10.3389/fmicb.2022.898744
Paddock KJ, Pereira AE, Finke DL, Ericsson AC, Hibbard BE, Shelby KS (2021) Host resistance to Bacillus thuringiensis is linked to altered bacterial community within a specialist insect herbivore. Mol Ecol 30:5438–5453. https://doi.org/10.1111/MEC.15875
Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375. https://doi.org/10.1146/annurev-phyto-082712-102340
Pineda A, Zheng S-J, van Loon JJA, Pieterse CMJ, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15:507–514. https://doi.org/10.1016/j.tplants.2010.05.007
Prasifka JR, Schmidt NP, Kohler KA, O’Neal ME, Hellmich RL, Singer JW (2006) Effects of living mulches on predator abundance and sentinel prey in a corn–soybean–forage rotation. Environ Entomol 35:1423–1431. https://doi.org/10.1093/ee/35.5.1423
Prischmann DA, Lehman RM, Christie AA, Dashiell KE (2008) Characterization of bacteria isolated from maize roots: Emphasis on Serratia and infestation with corn rootworms (Chrysomelidae: Diabrotica). Appl Soil Ecol 40:417–431. https://doi.org/10.1016/j.apsoil.2008.06.012
Quast C, Pruesse E, Yilmaz P et al (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41D:D590–D596. https://doi.org/10.1093/nar/gks1219
Riedell WE (1990) Rootworm and mechanical damage effects on root morphology and water relations in maize. Crop Sci 30:628–631. https://doi.org/10.2135/cropsci1990.0011183X003000030031x
Rivers A, Voortman C, Barbercheck M (2020) Cover crops support arthropod predator activity with variable effects on crop damage during transition to organic management. Biol Control 151:104377. https://doi.org/10.1016/j.biocontrol.2020.104377
Rowen EK, Tooker JF (2021) Ground predator activity-density and predation rates are weakly supported by dry-stack cow manure and wheat cover crops in no-till maize. Environ Entomol 50:46–57. https://doi.org/10.1093/ee/nvaa136
Russell VL (2022) emmeans: Estimated marginal means, aka least-squares means. R package version 1.7.2. https://CRAN.R-project.org/package=emmeans
Sadler EJ, Lerch RN, Kitchen NR et al (2015) Long-term agroecosystem research in the Central Mississippi River Basin: introduction, establishment, and overview. J Environ Qual 44:3–12. https://doi.org/10.2134/jeq2014.11.0481
Schmer MR, Jin VL, Wienhold BJ, Becker SM, Varvel GE (2020) Long-term rotation diversity and nitrogen effects on soil organic carbon and nitrogen stocks. Agric Ecosyst Environ 3:e20055. https://doi.org/10.1002/agg2.20055
Spike BP, Tollefson JJ (1991) Yield response of corn subjected to western corn-rootworm (Coleoptera, Chrysomelidae) infestation and lodging. J Econ Entomol 84:1585–1590. https://doi.org/10.1093/jee/84.5.1585
Tilman D, Isbell F, Cowles JM (2014) Biodiversity and ecosystem functioning. Annu Rev Ecol Evol Syst 45:471–493. https://doi.org/10.1146/annurev-ecolsys-120213-091917
USDA National Agricultural Statistics Service (2017) Census of Agriculture. USDA National Agricultural Statistics Service, Washington, DC. https://www.nass.usda.gov/Publications/Highlights/2020/census-land-use-practices.pdf. Accessed 26 Dec 2022.
van den Bosch TJM, Welte CU (2017) Detoxifying symbionts in agriculturally important pest insects. Microb Biotechnol 10:531–540. https://doi.org/10.1111/1751-7915.12483
Veum KS, Kremer RJ, Sudduth KA, Kitchen NR, Lerch RN, Baffaut C, Stott DE, Karlen DL, Sadler EJ (2015) Conservation effects on soil quality indicators in the Missouri Salt River Basin. J Soil Water Conserv 70:232–246. https://doi.org/10.2489/jswc.70.4.232
Veum KS, Zuber SM, Ransom C, Myers RL, Kitchen NR, Anderson SH (2022) Reduced tillage and rotational diversity improve soil health in Missouri. J Agron 114:3027–3039. https://doi.org/10.1002/agj2.21156
Wagner MR, Tang C, Salvato F, Clouse KM, Bartlett A, Vintila S, Phillips L, Sermons S, Hoffmann M, Balint-Kurti PJ, Kleiner M (2021) Microbe-dependent heterosis in maize. Proc Natl Acad Sci 118:e2021965118. https://doi.org/10.1073/pnas.2021965118
Wallander S, Smith D, Bowman M, Claassen R (2021) Cover crop trends, programs, and practices in the United States. Econ Inf Bull 222, U.S. Department of Agriculture, Economic Research Service. https://doi.org/10.22004/ag.econ.309562
Walsh CM, Becker-Uncapher I, Carlson M, Fierer N (2021) Variable influences of soil and seed-associated bacterial communities on the assembly of seedling microbiomes. ISME J 15:2748–2762. https://doi.org/10.1038/s41396-021-00967-1
Walters WA, Jin Z, Youngblut N et al (2018) Large-scale replicated field study of maize rhizosphere identifies heritable microbes. Proc Nat Acad Sci 115:7368–7373. https://doi.org/10.1073/PNAS.1800918115
Wechsler S, Smith D (2018) Has resistance taken root in US corn fields? Demand for insect control. J Agric Econ 100:1136–1150. https://doi.org/10.1093/ajae/aay016
Xue K, Serohijos RC, Devare M, Duxbury J, Lauren J, Thies JE (2012) Short-term carbon allocation and root lignin of Cry3Bb Bt and NonBt corn in the presence of corn rootworm. Appl Soil Ecol 57:16–22. https://doi.org/10.1016/j.apsoil.2012.02.014
Acknowledgements
The authors thank David Tague, Julie Barry, Amanda Ernwall, and Adriano Pereira for help with experimental set up. We thank Norman Best and Kate Guill for assistance with the large volume centrifuge, and Kent Shelby for providing materials and helpful feedback throughout the project. We acknowledge the efforts of Bioinformatics and Analytics Core and the Metagenomics Center at the University of Missouri. This work was supported by the USDA National Institute of Food and Agriculture Early Workforce Development Fellowship 2022-67011-36566, Hatch project #7002373 and USDA-ARS. This research was also a contribution from the Long-Term Agroecosystem Research (LTAR) network. LTAR is supported by the United States Department of Agriculture.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Hatch project #7002373 and EWD 2022-67011-36566. This research was also a contribution from the Long-Term Agroecosystem Research (LTAR) network. LTAR is supported by the United States Department of Agriculture.
Author information
Authors and Affiliations
Contributions
KJP collected and analyzed data. ACE provided sequencing support. KSV provided technical support throughout field work. BEH provided insect populations. KJP wrote the first manuscript draft and prepared the figures. All authors contributed to subsequent edits of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Christos Athanassiou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised: the figure 2 is revised.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paddock, K.J., Veum, K.S., Finke, D.L. et al. Soil microbes from conservation agriculture systems reduce growth of Bt-resistant western corn rootworm larvae. J Pest Sci (2024). https://doi.org/10.1007/s10340-023-01725-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01725-2