Abstract
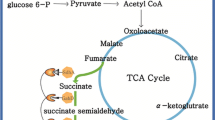
Gamma-aminobutyric acid (GABA) is an important bio-product, which is used in pharmaceutical formulations, nutritional supplements, and biopolymer monomer. The traditional GABA process involves the decarboxylation of glutamate. However, the direct production of GABA from glucose is a more efficient process. To construct the recombinant strains of Escherichia coli, a novel synthetic scaffold was introduced. By carrying out the co-localization of glutamate synthase, glutamate decarboxylase, and GABA transporter, we redirected the TCA cycle flux to GABA pathway. The genetically engineered E. coli strain produced 1.08 g/L of GABA from 10 g/L of initial glucose. Thus, with the introduction of a synthetic scaffold, we increased GABA production by 2.2-fold. The final GABA concentration was increased by 21.8 % by inactivating competing pathways.







Similar content being viewed by others
References
Conrado RJ, Wu GC, Boock JT, Xu H, Chen SY, Lebar T, Turnšek J, Tomšič N, Avbelj M, Gaber R, Koprivnjak T, Mori J, Glavnik V, Vovk I, Benčina M, Hodnik V, Anderluh G, Dueber JE, Jerala R, DeLisa MP (2012) DNA-guided assembly of biosynthetic pathways promotes improved catalytic efficiency. Nucleic Acids Res 40:1879–1889. doi:10.1093/nar/gkr888
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645. doi:10.1073/pnas.120163297
Delebecque CJ, Lindner AB, Silver PA, Aldaye FA (2011) Organization of intracellular reactions with rationally designed RNA assemblies. Science 333:470–474. doi:10.1126/science.1206938
Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KLJ, Keasling JD (2009) Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol 27:753–759. doi:10.1038/nbt.1557
Good MC, Zalatan JG, Lim WA (2011) Scaffold proteins: Hubs for controlling the flow of cellular information. Science 332:680–686. doi:10.1126/science.1198701
Tinoco I Jr, Sauer K, Wang JC, Puglisi JD, Harbison G, Rovnyak D (2002) Physical chemistry: principles and applications in biological sciences, 4th edn. Prentice Hall, Upper Saddle River, New Jersey
Jung YK, Kim TY, Park SJ, Lee SY (2010) Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol Bioeng 105:161–171. doi:10.1002/bit.22548
Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK (2000) Autoinhibition and activation mechanisms of the Wiskott–Aldrich syndrome protein. Nature 404:151–158. doi:10.1038/35004513
Kim SH, Shin BH, Kim YH, Nam SW, Jeon SJ (2007) Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol Bioprocess Eng 12:707–712. doi:10.1007/BF02931089
Moon TS, Dueber JE, Shiue E, Prather KLJ (2010) Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metab Eng 12:298–305. doi:10.1016/j.ymben.2010.01.003
Nguyen JT, Turck CW, Cohen FE, Zuckermann RN, Lim WA (1998) Exploiting the basis of proline recognition by SH3 and WW Domains: design of N-substituted inhibitors. Science 282:2088–2092. doi:10.1126/science.282.5396.2088
Oh CH, Oh SH (2004) Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. J Med Food 7(1):19–23. doi:10.1089/109662004322984653
Park KB, Ji GE, Park MS, Oh SH (2005) Expression of rice glutamate decarboxylase in Bifidobacterium Longum enhances γ-Aminobutyric acid production. Biotechnol Lett 27:1681–1684. doi:10.1007/s10529-005-2730-9
Park KB, Oh SH (2006) Enhancement of γ-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnol Lett 28:1459–1463. doi:10.1007/s10529-006-9112-9
Park SJ, Kim EY, Noh W, Oh YH, Kim HY, Song BK, Cho KM, Hong SH, Lee SH, Jegal J (2013) Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant Escherichia coli. Bioprocess Biosyst Eng 36(7):885–892. doi:10.1007/s00449-012-0821-2
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Saskiawan I (2008) Biosynthesis of polyamide 4, a biobased and biodegradable polymer. J Microbiol Indones 2(3):119–123
Schultz J, Hoffmuuller U, Krause G, Ashurst J, Macias MJ, Schmieder P, Schneider-Mergener J, Oschkinat H (1998) Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels. Nat Struct Mol Biol 5:19–24. doi:10.1038/nsb0198-19
Vo TD, Kim TW, Hong S (2012) Effects of glutamate decarboxylase and gamma-aminobutyric acid (GABA) transporter on the bioconversion of GABA in engineered Escherichia coli. Bioprocess Biosyst Eng 35:645–650. doi:10.1007/s00449-011-0634-8
Vo TD, Ko JS, Park SJ, Lee SH, Hong SH (2013) Efficient gamma-aminobutyric acid bioconversion by employing synthetic complex between glutamate decarboxylase and glutamate/GABA antiporter in engineered Escherichia coli. J Ind Microbiol Biotechnol 40:927–933. doi:10.1007/s10295-013-1289-z
Wu X, Knudsen B, Feller SM, Zheng J, Sali A, Cowburn D, Hanafusa H, Kuriyan J (1995) Structural basis for the specific interaction of lysine-containing proline-rich peptides with the N-terminal SH3 domain of c-Crk. Structure 3:215–226. doi:10.1016/S0969-2126(01)00151-4
Acknowledgments
This work was financially supported by grants from the Next-Generation BioGreen 21 Program (SSAC, Grant Number: PJ01111601), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dung Pham, V., Somasundaram, S., Lee, S.H. et al. Efficient production of gamma-aminobutyric acid using Escherichia coli by co-localization of glutamate synthase, glutamate decarboxylase, and GABA transporter. J Ind Microbiol Biotechnol 43, 79–86 (2016). https://doi.org/10.1007/s10295-015-1712-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1712-8