Abstract
Mexico is a major center of evolutionary radiation for the genus Quercus, with oak species occurring across different habitat types and showing a wide variation in morphology and growth form. Despite representing about 20% of Mexican species, scrub oaks have received little attention and even basic aspects of their taxonomy and geographic distribution remain unresolved. In this study, we analyzed the morphological and climatic niche differentiation of scrub oak populations forming a complex constituted by six named species, Quercus cordifolia, Quercus frutex, Quercus intricata, Quercus microphylla, Quercus repanda, Quercus striatula and a distinct morphotype of Q. striatula identified during field and herbarium work (hereafter named Q. striatula II). Samples were obtained from 35 sites covering the geographic distribution of the complex in northern and central Mexico. Morphological differentiation was analyzed through geometric morphometrics of leaf shape and quantification of trichome traits. Our results indicated the presence of two main morphological groups with geographic concordance. The first was formed by Q. frutex, Q. microphylla, Q. repanda and Q. striatula, distributed in the Trans-Mexican Volcanic Belt, the Sierra Madre Occidental and a little portion of the south of the Mexican Altiplano (MA). The second group consists of Q. cordifola, Q. intricata and Q. striatula II, found in the Sierra Madre Oriental and the MA. Therefore, our evidence supports the distinctness of the Q. striatula II morphotype, indicating the need for a taxonomic revision. Within the two groups, morphological differentiation among taxa varied from very clear to low or inexistent (i.e. Q. microphylla-Q. striatula and Q. cordifolia-Q. striatula II) but niche comparisons revealed significant niche differentiation in all pairwise comparisons, highlighting the relevance of integrative approaches for the taxonomic resolution of complicated groups such as the one studied here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Quercus (Fagaceae) is recognized for its economic and ecological importance and recently has become a model clade in different fields of biology (Cavender-Bares 2019). For these reasons, defining and properly differentiating species in this group is a fundamental task (Valencia-A 2020; Wu et al. 2023). On the basis of phylogenomic analyses (Hipp et al. 2018, 2020), Quercus has recently been divided (Denk et al. 2017) into two subgenera with eight sections (subgenus Quercus, including sections Lobatae, Ponticae, Protobalanus, Quercus and Virentes, and subgenus Cerris, including sections Cerris, Cyclobalanopsis and Ilex). Mexico is recognized as a major center of diversification of the oaks, with more than 160 species, included in sections Lobatae, Quercus, Protobalanus and Virentes, and at least 109 endemics (Galicia et al. 2015; Hipp et al. 2018; McCauley et al. 2019; Nixon 1993; Valencia-A 2004).
Despite these recent advances in the clarification of the taxonomy and phylogeny of oaks, considerable confusion remains within groups of closely related species, especially in Mexico (Hipp et al. 2020). Some of these difficulties in the delimitation and identification of oak species arise from phenotypic similarity due to recent divergence, the presence of few taxonomically informative characters in floral traits and the great variation in vegetative characters at different levels (from intraindividual to between populations) (González-Rodríguez et al. 2004; Hardin 1975; Morales-Saldaña et al. 2022; Valencia-A 2004, 2020). Leaves usually display considerable intraspecific variation, since their morphology depends on an intricate relationship between genetic and environmental factors (Chitwood and Sinha 2016; De Heredia et al. 2018). Regarding genetic factors, the frequent gene flow among oak species impacts the expression of morphological characters (Cannon and Petit 2020; Dumolin-Lapégue et al. 1999; Leroy et al. 2017; Whittemore and Schaal 1991). In turn, variation in factors such as light exposure, water availability or temperature, and even pruning, may result in leaf differences between individuals of the same population or between branches of the same individual (Aykut et al. 2017; Borazan and Babaç 2003; Jensen 1990). Therefore, the high degree of leaf polymorphism in oak trees results from the interrelation between introgressive hybridization, phenotypic plasticity, and adaptation to environmental gradients (Morales-Saldaña et al. 2022). In addition to the large intraspecific variation, many of the vegetative characters in Quercus also show low interspecific differentiation or tend to evolve in a convergent or parallel manner (Jones 1986; Tucker 1974).
In Mexico, some oak species complexes have been analyzed to demarcate taxa, either from a strictly morphological perspective, as for the Acutifoliae group (Romero Rangel 2006) or, more recently, using integrative approaches, as for the Quercus laeta complex (Morales-Saldaña et al. 2022) and the Racemiflorae group (McCauley et al. 2019). In these recent studies, quantitative methods for analyzing vegetative characters have proven useful for the identification of differentiated groups. Such methods include geometric morphometrics (Albarrán-Lara et al. 2010; Morales-Saldaña et al. 2022; Rodríguez-Gómez et al. 2018; Viscosi 2015; Viscosi et al. 2009b), as well as the statistical characterization of micromorphological structures that are considered of high taxonomic value, such as trichomes (Fortini et al. 2015; López-Caamal et al. 2017).
In comparison to their arboreal congeners, less attention has been given so far to most of the approximately 70 scrub oak species that exist worldwide. At least 31 species with this growth form are distributed in Mexico (21 in section Quercus, seven in Lobatae and three in Protobalanus); 20 of them are endemic and 11 are shared with the USA. In California, studies of scrub oak species have revealed that they frequently form hybrid swarms with adaptations to local environmental conditions, which represents an interesting scenario for the origin of new species (Ortego et al. 2015; Sork et al. 2016). In the case of Mexico, Sabás-Rosales et al. (2017) provided a qualitative analysis of the leaf morphology and trichome types of six scrub oak species. These six species are part of the Q. microphylla Née complex, in which all taxa are strictly shrubby in habit, show elliptic, ovate, oblong or lanceolate leaves, mostly with stellate trichomes on the abaxial side or with some stipitate or fasciculate trichomes. Intergradation in leaf morphology is evident among these species (Sabás-Rosales et al. 2017) and, in addition, their area of distribution is not clearly known, mainly because the original descriptions are not sufficiently precise in terms of diagnostic characters, which possibly has caused numerous misidentifications and, consequently, the over- or underestimation of the real distribution of the taxa.
The complex analyzed in this study is named after Q. microphylla, a scrub oak species with broad distribution in Mexico (González-Elizondo et al. 1993; Romero Rangel et al. 2002; Sabás-Rosales et al. 2017; Valencia-A 2004; Valencia-A et al. 2017; Vázquez-Villagrán 2000) and problematic taxonomically. The synonymies reported for Q. microphylla involve putative varieties of Q. frutex Trel. (Valencia-A 2004) and Q. striatula Trel. (Bartholomew and Almeda 2023; González Villarreal 1986; McVaugh 1974), problems that have produced a “domino” effect since the inconspicuous differences and apparent sympatric distribution between Q. microphylla and Q. striatula involve Q. cordifolia Trel. too (Muller 1944; Villareal et al. 2008). In the same way, Q. frutex may show confusion with Q. repanda Bonpl. in distribution and appearance (Romero Rangel et al. 2002, 2014). Quercus intricata Trel. adds to the problem by sharing characters such as leaf texture and indumentum appearance with Q. frutex and Q. repanda, in addition to presenting a sympatric distribution with Q. cordifolia. Finally, the complex also includes Quercus deliquescens and Quercus mohoriana. However, these are geographically restricted species in Mexico, with a distinguishable phenotype from the rest of the complex (Muller 1944; Sabás-Rosales et al. 2017).
In this context, the main objective of this study was to contribute to the recognition of six scrub oak species belonging to the Q. microphylla complex (Q. cordifolia, Q. frutex, Q. intricata, Q. microphylla, Q. repanda and Q. striatula) by: (i) describing the leaf morphology of each species, from a geometric morphometrics approach, (ii) characterizing the types of trichomes present in the species, as well as evaluating if there are differences in the number of trichome arms among the species, and (iii) defining the potential areas of distribution and assessing whether there are niche differences among the species of the Q. microphylla complex.
Materials and methods
Study species
The six species in this study belong to the section Quercus (withe oaks). The six species are shrubby in habit, with rhizomatous growth and with small (0.5 to 2.6 cm) and tomentose to glabrescent leaves. Except for Q. intricata, which is also distributed in the southern USA, the rest of the species are endemic to Mexico.
Quercus cordifolia Trel. (Fig. 1) grows as clearly differentiated individuals with a stem of 0.5 to 1 m in height. The leaves of this species are papyrose, ovate, ovate-lanceolate or ovate-elliptic with a generally cordate base and rounded to acute apex, with a flat and entire or sparsely lobed margin. The underside can be from dense tomentose to glabrescent, with the tomentum formed by stellate trichomes. The species is found at elevations of 1700 to 3200 m, in pine and pine-oak forests in the north of the Sierra Madre Oriental (Sabás-Rosales et al. 2017; Valencia-A 2004).
Quercus frutex Trel. (Fig. 1) forms extensive patches, with stems measuring 0.6 to 1.5 m. The leaves of the species are elliptic-oblong or oblong-lanceolate with a rounded base and rounded to acute apex. The texture of the leaves is subcoriaceous, with entire and revolute margins. The underside is tomentose with stipitate-fasciculate trichomes. It occurs in the clearings of oak, pine-oak, pine or fir forests and can be the dominant species in the Quercus scrub of the Trans-Mexican Volcanic Belt between 2000 and 3000 m. (Valencia-A et al. 2017; Vázquez-Villagrán 2000).
Quercus intricata Trel. (Fig. 1) can measure from 0.4 to 1.5 m in height. This species has ovate, ovate-elliptic or elliptic-oblong leaves with rounded to slightly cordate base and acute or rounded apex. Leaf texture is coriaceous, margins are serrated and notoriously revolute. Leaf undersides are densely pubescent with stellate trichomes. This species is generally distributed in the periphery of forests at the base of mountains or in dry thickets. It is an important part of submontane scrubs, in the transition zones with rosetophylous scrub and stone pine forest, on stony soils. It is found at elevations from 1600 to 2700 m in the states of Coahuila, Nuevo León and Chihuahua (Sabás-Rosales et al. 2017; Valencia-A 2004; Villareal et al. 2008).
Quercus microphylla Neé (Fig. 1) has several erect stems of 0.2 to 0.7 m in height emerging from the rhizome, making it difficult to delimit individuals. The species grows in dense but not large patches. The leaves are elliptic-lanceolate or oblong-lanceolate with rounded or obtuse base, acute to slightly rounded apex, subcoriaceous texture, flat to slightly revolute margin and tomentose to slightly glabrescent underside with stellate trichomes. It is found in xerophytic shrublands and pine forests, on limestone soils or less frequently on rocky soils, at elevations between 2200 and 2500 m. It is known from the center and east of the Trans-Mexican Volcanic Belt, from the states of Guanajuato, Hidalgo, Puebla, Tlaxcala and Veracruz (Valencia-A 2004; Vázquez-Villagrán 2000).
Quercus repanda Bonpl. (Fig. 1) also has several erect stems of 0.2 to 1.5 m in height that emerge from the rhizome, making it difficult to delimit individuals. Leaves are obovate or elliptic-oblong, with a cuneate to rounded base and obtuse to rounded apex, coriaceous texture and revolute-repanding margin. The underside is tomentose with sessile fasciculate trichomes. It grows as small islands in the clearings of pine and pine-oak forests and as a scarce element in xerophytic shrublands, at elevations of 2,100 to 3,140 m. It is mainly distributed in the Trans-Mexican Volcanic Belt, in the states of Guanajuato, Hidalgo, Michoacán, Puebla, Querétaro, San Luis Potosí and Veracruz (Valencia-A et al. 2017).
Quercus striatula Trel. (Fig. 1) is also characterized by erect stems of 0.3 to 1.2 m in height that emerge from the rhizome. It grows as scattered islands and covers large surfaces at the edges of forests or in clearings. It has elliptic-lanceolate or oblong-lanceolate leaves with rounded to obtuse base and acute to rounded apex. The texture of the leaves is subcoriaceous and the margin is flat to slightly revolute with tomentose undersides with stellate trichomes. It is generally associated with xerophilous scrub or pine forests, between 2,100 and 3,000 m of elevation. It is distributed on the inner slopes of the Sierra Madre Occidental, in the states of Chihuahua and Durango (Sabás-Rosales et al. 2017).
Collection of botanical material and grouping of populations
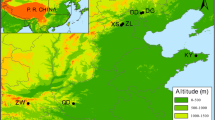
To determine the distribution of the species and to select the collection localities for the different populations, we revised the specimens determined under the names Q. cordifolia, Q. frutex, Q. intricata, Q. microphylla, Q. repanda and Q. striatula deposited in the National Herbarium of Mexico (MEXU) and the herbarium of the Faculty of Sciences (FCME), both belonging to the National Autonomous University of Mexico (UNAM) (Thiers 2016). Specimens that had geographic coordinates and concurred with the original descriptions of the species and with the type specimens consulted in the electronic resource JSTOR Global Plants (plants.jstor.org) were selected. From this survey, 35 localities were chosen and sampled for the six species (Fig. 2, Table S1); also taking into account accessibility and safety aspects. During the field work, in some populations we observed variations in growth form and leaf morphology, texture and indumentum, characteristics that deviated from those reported in the original descriptions of Q. striatula, so these populations were designated as a variant “morphotype” (hereafter called Q. striatula II) (see Fig. 1). For each of the collected populations, five leaves per individual (from different parts of the shrub) were selected for all sampled individuals at the population (5–20), ensuring that selected leaves were mature and without noticeable herbivory marks. Subsequently, the leaves were pressed and dried.
Collection sites of the populations of the Q. microphylla complex, accompanied by the population number according to Table 1. Gray shading indicates elevation (m a.s.l.)
Morphological analyses
Geometric morphometrics. Once dried, leaves were digitized using a flatbed scanner (Hp SJ300) at a resolution of 300 dpi, always accompanied by a size scale. In order to represent the leaf shape, we placed two landmarks at homologous points of each leaf (the insertion of the petiole with the lamina, and the apex), as well as 35 equidistant points on the leaf outline (Fig. S1). Then, the tpsDig2.10 (Rohlf 2006) software was used to generate a data matrix (x, y coordinates of each of the 37 total landmarks) in “.TPS” format.
From the “.TPS” file, the matrix of coordinates was imported into the Geomorph 4.0.3 (Adams et al. 2016) package of the R Statistical Software (v4.1.2; R Core Team 2021), where the first and last points of each leaf outline were removed to avoid overlap with the apical and basal landmarks. Subsequently, a Generalized Procrustes Analysis (GPA) was implemented to remove size, position, and rotation effects. From the resulting datasets, leaf shape was averaged for each individual using the mshape function of the Geomorph package. In order to visualize the clustering patterns of individuals while maximizing the variance between a priori defined groups (i. e. the six species plus the Q. striatula II morphotype), a canonical variate analysis (CVA) was performed using the CVA function implemented in the Morpho 2.11 package (Schlager 2017). Additionally, subsequent CVAs were performed to explore differences within the main groups of individuals segregated by the previous analysis, this time averaging leaf shape for each population. Finally, linear distances between different pairs of landmarks (i. e. blade length, middle blade width, apical width and basal width) were calculated (Fig. S2), to analyze in a traditional way the leaf morphological differences between the taxa, using the interlmkdist function of the Geomorph package. Boxviolin diagrams were used to represent values of each of these linear distances, grouped by taxon. Differences among taxa were evaluated using the Kruskall-Wallis test, followed by a Dunn test as a post-hoc test (due to the non-normality of the data), implemented in the R software.
Trichome imaging. Trichomes were observed in two randomly chosen individuals from each population collected. Trichomes were scraped from the abaxial side, always from the central part of the leaf blade in the region between secondary veins and mounted on a microscope slide using fast-drying nail polish. Subsequently, these preparations were observed under an optical microscope (Zeiss Axio Imager.A2) with a 10 × objective, and images of the trichomes were digitalized and scaled using an integrated photographic camera for further analysis. From each preparation, ten trichomes were subsequently randomly selected, considering both zenithal and lateral views, to clearly observe the number of arms and their pattern of insertion. Trichomes were characterized according to the different types proposed by Jones (1986). In addition, the number of arms of each trichome was counted and a Kruskall-Wallis test, followed by a Wilcoxon test, was applied to evaluate possible differences between taxa, using the R software.
Ecological niche models
Ecological niche models (ENM) were constructed considering seven entities: i. e. the six species and the Q. striatula II morphotype (Table S2). Presence records for each entity were obtained from our own field collections and specimen data from FCME and MEXU herbaria; the final dataset consisted of 15 records for Q. cordifolia, 14 for Q. frutex, 31 for Q. intricata, five for Q. microphylla, 13 for Q. repanda, 11 for Q. striatula, and 27 records for the Q. striatula II morphotype. To avoid overfitting the models, the thinning function of the spThin package (Aiello-Lammens et al. 2015) in R software was used, which randomly selects presence points separated by at least 5 km, using 100 replicates.
The 19 bioclimatic variables were obtained from the Worldclim dataset at a spatial resolution of 30 arcseconds (Fick and Hijmans 2017); https://worldclim.org/data/worldclim21.html) for the area corresponding to Mexico. These layers were trimmed into smaller areas to be used in the analyses for each taxon. The criterion for trimming was to reduce the layers according to the accessible area (area M) for each taxon, which was defined based on the ecoregions file of the World Wildlife Fund (Olson et al. 2001), selecting those ecoregions that coincided with the location of the collection sites for each taxon, as well as the areas contiguous to them, where they could hypothetically be present (Martinez-Meyer 2005). Thus, the resulting areas were used as a “clip” to trim the set of bioclimatic layers that were used to elaborate the ENM for each taxon. The selection and editing of the ecoregions, as well as the clipping and transformation of the bioclimatic layers to ASCII format, were performed using the QGis 3.8.3 program (QGIS Development Team 2019).
Because using a large number of bioclimatic layers can lead to overfitting of the models (Peterson and Nakazawa 2008), a pre-selection of bioclimatic variables was performed before making the final ENM. Initially, the 19 climatic variables were considered to obtain the distribution model for each taxon. From the models obtained and based on the Jackknife test of MaxEnt ver. 3.4.1 k (Phillips et al. 2006), those variables with the highest percentage of contribution to the model for each taxon were selected. Subsequently, by means of a Pearson correlation analysis in R software, from each pair of highly correlated variables (r > 0.80) one was eliminated, thus obtaining the sets of variables for each of the taxa (Table S2).
The ENM were performed with the maximum entropy algorithm in Maxent version 3.4.1 k (Phillips et al. 2006), since it has been demonstrated with different simulations that this algorithm generates good predictions, even with a reduced number of records (< 10; Pearson et al. 2007; Phillips et al. 2006). The parameters for the development of the models were those that came by default in the program (Phillips and Dudík 2008), except for the Extrapolate and Do clamping options that were deactivated to avoid artificial extrapolations in the extreme values of the ecological variables (Elith et al. 2011). The model output format was logistic, and 75% of the records was used to train the models and the remaining 25% to validate them; this partitioning was done randomly. Initially, the area under the curve (AUC) value of the receiver operating characteristic (ROC) was used to evaluate the robustness of the ENM. Those models with AUC values between 0.8–0.9 were considered reasonably good and ENM with values above 0.9 were categorized as very good (Peterson et al. 2011). However, considering that the usefulness of ROC analysis has been questioned (Lobo et al. 2008; Peterson and Nakazawa 2008), the final ENM were also validated by partial ROC analysis, which has been designed to address the shortcomings of ROC analysis (Peterson et al. 2008). This analysis was performed using the online tool NicheToolBox (http://shiny.conabio.gob.mx:3838/nichetoolb2/) with 1000 replicates and an omission ratio (E) = 0.05.
Finally, the degree of climate niche differentiation among taxa was measured via Schoener's D (Schoener 1968), using the niche similarity test implemented in ENM Tools (Warren et al. 2010, 2021). This test is recommended when species to be compared are partially or fully allopatric (Warren et al. 2010, 2021) and employs randomization to compute a null distribution and estimate whether the ENMs from different species are more or less like each other than expected by chance, based on environmental differences in the environment in which they occur (Warren et al. 2008). The test was run using the “background.test” function in R package “ENMTools” (Warren et al. 2010, 2021) with 100 replicates, sampling a total of 10,000 random points and was analyzed with a two-tailed test.
Results
Geometric morphometrics
The canonical variate analysis among all individuals indicated that 75.3% of the total variance in leaf shape was explained by the first two axes (CV1 = 53.7%, CV2 = 21.6%). The scatter plot of the first two canonical variates revealed that it is possible to distinguish two main groups separated along the first axis (Fig. 3). These groups differ significantly according to the calculated Mahalanobis distances between taxa (Table 1). The first of these two groups (Group I) is situated at negative values of the first axis and is formed by individuals of Q. frutex, Q. microphylla, Q. repanda and Q. striatula, all of which possess elliptic-oblong leaf morphologies. Meanwhile, the second group (Group II) is located towards positive values of the same axis and included individuals of Q. cordifola, Q. intricata and Q. striatula II, characterized by ovate leaves with a slightly cordate base (Fig. 3). Interestingly, these groups have a geographic correspondence. Populations composing Group I are distributed mainly on the Trans-Mexican Volcanic Belt (TMVB), the Sierra Madre Occidental (SMOc) and a little portion of the south of the Mexican Altiplano (MA), while populations in Group II are mainly found over the Sierra Madre Oriental (SMOr) and the Mexican Altiplano (MA) (Fig. 2).
Subsequent canonical variate analysis for the population averages of the samples belonging to Group I explained 88.7% of the variance on the first two axes (CV1 = 58%, CV2 = 30.7%). The scatter plot suggested two main morphological groupings within this group, one formed by Q. frutex and Q. repanda (with negative values along the CV1), with elliptic-oblong leaves with a slightly attenuated base. On the other hand, Q. microphylla and Q. striatula (with positive values along the CV1) formed another grouping with predominantly elliptic blades with a rounded base (Fig. 4a). However, Q. frutex-Q. repanda and Q. striatula-Q. microphylla did not differ significantly according to Mahalanobis distances (Table 2).
Likewise, in the case of Group II, the CVA analysis recovered 99% of the variation in the first two axes (CV1 = 74.1%, CV2 = 25.1%). In this case, it segregated the populations of Q. intricata, characterized by elliptic-ovate forms with attenuated apex and slightly cordate base, with respect to a second morphological group formed by Q. cordifolia and the Q. striatula II morphotype, both with ovate leaf blades with cordate base (Fig. 4b). In this case, the comparison between Q. cordifolia and the Q. striatula II morphotype was not significant in terms of Mahalanobis distances (Table 2).
The distribution of the values obtained for the linear distances between pairs of landmarks reaffirms the distinctness of most taxa in pairwise comparisons for each variable (Fig. 5). Despite the similarities in leaf shape among the taxa within each of the two morphological groups, the comparisons of their linear dimensions were significant in most cases. In general, the pair of taxa with more discrimination difficulties with these variables is Q. cordifolia-Q. intricata which showed the least differences (Fig. 5).
Box-violin plots of linear distances: a blade length, b middle blade width, c basal width, d apical width. Colors correspond to the two main morphological groups in Fig. 3, with red indicating taxa included in Group I and blue indicating taxa in Group II. Different letters indicate significant differences among groups according to a Kruskal–Wallis test followed by a Dunn test. Q. f = Q. frutex, Q. m = Q. microphylla, Q. r = Q. repanda, Q. s = Q. striatula, Q. c = Q. cordifolia, Q. i = Q. intricata, Q. s II = Q. striatula II
Trichome analysis
Stellate-type trichomes were found in all populations analyzed, with presence of stipitate fasciculate ones in Q. frutex and very scarcely in Q. intricata and Q. microphylla (Fig. 6). The number of arms of the trichomes ranged from five to 23 with a mean of eight trichomes for most taxa. The Kruskall-Wallis test (χ2 = 81.217, P = 0.0001) followed by a Wilcoxon post-hoc test indicated significant differences in arm number between Q. cordifolia-Q. striatula, Q. microphylla-Q. striatula and Q. repanda from all the other taxa (Fig. S3).
Ecological niche models
From the Jackknife analysis and Pearson’s test, a total of two (Q. cordifolia and Q. repanda) to seven (Q. striatula II) non-collinear bioclimatic variables were chosen to perform the ENM of the different taxa (Table S2). In general, the ENM for the analyzed taxa were categorized as “very good” based on the AUC values, which were greater than 0.9, except for the model of Q. repanda, which had AUC = 0.89 for the training data and AUC = 0.86 for the validation data and was thus considered as “reasonably good”. Likewise, partial ROC tests yielded AUC ratios with values significantly (P < 0.0001) greater than 1 (Table S3), indicating that the models obtained are statistically better than chance.
The potential distribution maps based on the ENM showed three main geographical patterns: (i) taxa with higher suitability in the TMVB (Q. frutex, Q. microphylla and Q. repanda); (ii) taxa with higher suitability in the SMOc (Q. striatula); and (iii) taxa with higher suitability in the SMOr and MA (Q. cordifolia, Q. intricata and Q. striatula II) (Fig. 7). These patterns also coincided with the vegetation type where the different taxa were collected: oak forest margins (TMBV), pine-oak forest clearings (SMOc), scrub and pine-oak forest (SMOr), and xerophytic scrub with pine (MA).
Potential distribution of seven taxa of Mexican shrub oaks. a Q. frutex, b Q. cordifolia, c Q. microphylla, d Q. intricata, e Q. striatula, f Q. striatula II, g Q. repanda and h distribution of two main morphological groups (red = morphological Group I; blue = morphological Group II). Gray shading indicates elevation (m a.s.l)
Compatible with what was observed with the morphological groupings, Q. frutex, Q. microphylla and Q. repanda (Fig. 7a, c, g) are codistributed in some parts of the TMBV, particularly towards the central part. However, the distribution of Q. frutex and Q. repanda is mostly located towards the east, while Q. microphylla shows a fragmented distribution mainly towards the western part of the TMBV. The fourth species of this group, Q. striatula, shows a potential distribution restricted to the SMOc (Fig. 7e). On the other hand, the species in the morphological Group II showed similar potential distribution areas. Quercus striatula II (Fig. 7f) showed a discontinuous distribution in the highlands of Nuevo León state and southern Coahuila state, similar to what was observed for Q. cordifolia (Fig. 7b). Quercus intricata (Fig. 7d), although sympatric with these two previous species in some areas of the SMOr, is also found discontinuously in central and northern Coahuila and even reaches the United States of America, according to records not used in this work. The pairwise niche comparison tests were significant in all cases, with Schoener’s D values ranging between 0.069 and 0.688 (Table 3). The lowest D value was obtained between Q. striatula and Q. striatula II, revealing that the niche occupied by the two taxa are highly divergent from each other (D = 0.069; P = 0.009). In turn, the highest niche similarity was observed between Q. frutex and Q. repanda (D = 0.688: P = 0.029).
Discussion
Discrimination and circumscription of species at the morphological and molecular level, as well as their biogeographical and ecological characterization, is one of the current challenges prevailing for several species groups within the genus Quercus (Denk et al. 2017). However, this challenge is increased in groups of recently diverged species (Pinheiro et al. 2018), being particularly complicated when diversification or lineage formation occurs with few morphological changes (cryptic or sister species), or when reduced differentiation results from non-adaptive radiation, morphological stasis, or phenotypic convergence (Bickford et al. 2007; Morales-Saldaña et al. 2022; Nosil and Feder 2012; Valencia-A et al. 2016; Valencia-A 2020).
Leaf analysis from a population perspective, the first part of the puzzle
Recently, the need to adopt methodologies for taxonomic purposes based on data from several individuals and different populations has been highlighted, mainly due to the variation that individuals or populations may present (Di Pietro et al. 2020). Such variation arises as a result of phenotypic plasticity or local adaptation to the different environments in which populations develop, sometimes leading to the formation of ecomorphotypes, potentially causing a taxonomic overestimation of the number of species (Di Pietro et al. 2020; Proietti et al. 2021). At the other extreme, the number of species can be underestimated because of morphological similarities, as reported by Morales-Saldaña et al. (2022) for the Q. laeta complex, with a large number of morphotypes and synonymies within it, which may correspond to different species.
For this reason, among the most powerful tools of current morphological analysis, which has been used in different taxonomical complexes, geometric morphometrics has the advantage of providing statistical robustness, while the analysis of the shape as a whole will also implicitly evaluate different traits quantified by traditional methods (e.g., leaf length, width, distance between lobes and/or veins). In this way, a detailed analysis of the species hypotheses being tested is possible, including the identification of shape aspects for which the evaluated groups differ the most, therefore contributing to the elaboration of an overall diagnosis for each group of samples from a population perspective (Pérez-Pedraza et al. 2021; Valencia-Cuevas et al. 2015; Viscosi et al. 2009a, 2010; Yang et al. 2022).
The Q. microphylla complex has been reviewed in few taxonomic works. Trelease (1924) proposed the Microphyllae series, at that time only consisting of the currently recognized Q. microphylla, Q. frutex and Q. repanda. However, the high intraspecific morphological variation of these species generates a morphological gradation that results in an overlap of the forms reported in the original descriptions that, together with the presence of non-glandular trichomes in the indumentum of all of them, has caused diverse interpretations on the validity of the taxa (Sabás-Rosales et al. 2017).
Currently, the Q. microphylla complex as proposed by Valencia-A (2020) is composed of Q. cordifolia, Quercus deliquescens, Q. frutex, Q. intricata, Q. microphylla, Quercus mohoriana, Q. repanda and Q. striatula. Our geometric morphometrics results subdivided the complex into two groups (Fig. 3). On the one hand, in Group I, we found Q. frutex, Q. repanda, Q. microphylla and Q. striatula, for which the possible synonymy and identity problems between the latter and Q. cordifolia, previously discussed by Muller (1944); Martínez (1977) and Villareal et al. (2008), are morphologically ruled out. On the other hand, morphological similarity between Q. frutex and Q. repanda was observed (Fig. 4a), as reported by Romero et al. (2002) and Valencia-A et al. (2017). However, they differ in all linear dimensions and also in shape, from elliptic forms with attenuated base (Q. frutex) to obovate forms (Q. repanda) (Figs. 4a, 5). Finally, in this group there is similarity between Q. microphylla and Q. striatula, both with elliptical forms and rounded base, which may explain what was reported by McVaugh (1974) and González (1986).
On the other hand, it should be noted that the morphotype Q. striatula II, which has been determined as Q. striatula in herbaria, clearly differs from the type and specimens considered as Q. striatula in this work, partially explaining the identity problems between these taxa. In this regard, within Group II, the CVA based on population morphological averages clearly separated Q. intricata from Q. striatula II. At the same time, similarity was observed between the latter and Q. cordifolia (Fig. 4b), which have had identity problems according to Muller (1944) and Villareal et al. (2008) by presenting ovate leaves with a cordate base. Despite this, they differ in the four linear dimensions, with all measures being smaller in Q. striatula II (Fig. 5). So, apparently the taxonomic complexity of Q. cordifolia has actually occurred with Q. striatula II and not with Q. striatula.
Finally, the indumentum in all species presented trichomes of the stellate type, with a mean around eight arms for most of them (Q. cordifolia, Q. frutex, Q. intricata, Q. microphylla, Q. striatula and Q. striatula II) clearly separating only Q. repanda (mean of 19 arms) from the rest. In addition, the stipitate stellate trichome type is generally present in Q. frutex (Fig. 6). Therefore, these two taxa can be clearly discriminated from the rest of the complex under these two traits. Likewise, the number of arms achieved significant differences between Q. microphylla and Q. striatula, species with great similarity in morphometric measurements (Fig. 5). In this sense, it is still necessary to quantitatively evaluate the density of trichomes in the leaf lamina, as well as the epidermis type to find out if there are differences among the different taxa (Fortini et al. 2015; López-Caamal et al. 2017; Scareli-Santos et al. 2013; Tschan and Denk 2012).
Ecological and geographical patterns within the Q. microphylla complex
The geographic distribution of the taxa in the Q. microphylla complex is one aspect of notorious disagreement among different authors (González-Elizondo et al. 1993; Romero Rangel et al. 2002; Sabás-Rosales et al. 2017; Valencia-A 2004; Valencia-A et al. 2017; Vázquez-Villagrán 2000). From a total of 21 pairwise comparisons of potential distribution models, in six comparisons (28.6%) we found some degree of overlap in the geographic ranges of the taxa, while in 15 comparisons (71.4%) they showed non overlapping distributions (Table 3). This suggests that, in most cases, allopatry is the predominant distribution pattern among species of the Q. microphylla complex. In addition, when we contrasted the potential distributions between the two main groups detected through geometric morphometry, it was also possible to observe that these two groups are largely allopatric. Species of Group I are distributed towards the center and west of the country, through the TMVB, SMOc and the southern portion of the MA, while taxa in Group II are found mainly towards the center and northeast of Mexico, in the south and north of the MA and the SMOr (Fig. 7). Therefore, this zoning, together with morphometric analyses, allows an approximation of the identity of species from their distribution, making it possible to rule out the presence of some taxa in certain regions.
Complementarily, the background test allowed us to quantify the degree of niche similarity among taxa and to incorporate ecological evidence to clarify taxonomic controversies within the Q. microphylla complex. Firstly, the climatic niche comparisons allowed us to support the hypothesis that Q. striatula and Q. striatula II actually represent two distinct biological entities, with a remarkable morphometric differentiation, a clear allopatric distribution pattern and a strong niche divergence. Thus, they could be considered as two different species. In addition, in cases in which there is a limited morphometric differentiation, as it happens between Q. microphylla and Q. striatula, the analysis indicated a high degree of niche differentiation with an evident allopatric pattern, so that the low morphological differentiation could be due to phenotypic convergence, as has been reported for other Mexican oaks (Valencia-A 2020). Even though our results indicated a tendency toward niche divergence among taxa within the Q. microphylla complex, examples of taxa with high degree of niche similarity were also observed, which were accompanied by morphological similarity (e. g., Q. cordifolia-Q. striatula II, Q. frutex-Q. repanda and Q. microphylla-Q. repanda). However, the different types of evidence combined allows discrimination among taxa in almost all cases.
Finally, paradoxically Q. microphylla is the species of the complex with the widest previously reported distribution. In spite of this, during our field work it was very difficult to find populations of this species, so apparently, the high taxonomic complexity of the group has resulted in an overestimation of the distribution of this species, mainly towards the north of the country. In addition, one of the problems detected during the herbarium revision is that several specimens were indicated to have a tree growth form in the collection data. This alternation between tree and shrub habit can occur in species such as Quercus opaca or Quercus grisea. However, the species that comprise the Q. microphylla complex are strictly shrubby. But, apparently the shape, and particularly the size of the leaves, generates this confusion at the moment of identifying specimens, contributing to overestimate the distribution of the species.
Q. microphylla, Mexico’s largest shrub oak complex
The Q. microphylla complex represents the shrub oak complex with more species in Mexico (eight of at least 32 species present in the country). In addition to the species studied in the present work, Q. deliquescens and Q. mohriana are part of the complex, since they share the characteristics of shrubby habit, stellate trichomes and elliptic-oblong to ovate leaves. In the case of Q. deliquescens, it was not possible to locate any population corresponding to the specimens deposited in the herbaria, besides being presumably restricted to the state of Chihuahua. In turn, Q. mohriana is mainly distributed in the United States of America and in Mexico it is only found in the north of the state of Coahuila. Therefore, both species were not considered for the present work. However, all these species together result in a set of taxa with high morphological/taxonomic complexity within the genus Quercus.
Moreover, our results provide valuable information that can complement the poorly detailed descriptions of most species. And, consequently, they can serve as a basis for different types of studies in the genus. In this sense, it has been suggested to carefully analyze different evidence simultaneously (e. g. morphometric, ecological niche modeling, molecular and chemical data, etc.), with a wide representation of individuals and populations; and in this way, to assign a set of morphological characters covering the known distribution of the species to also assign its locus classicus (name linked to a type specimen, also linked to a geographical locality), as a way of adding additional ecological information enabling further identity of the species (Di Pietro et al. 2020).
Finally, the need for molecular analysis of species boundaries was highlighted. On the one hand, the strong separation between the morphotypes of Q. striatula suggests the presence of two different species (they are morphologically, geographically and ecologically distant). Therefore, the morphotype Q. striatula II may remain under some synonymy or be undescribed. On the other hand, Q. microphylla and Q. striatula are morphologically grouped, and show intraspecific variation that deviates from the description of each species, mainly in aspects such as leaf consistency, appearance of the indumentum and the way in which the shrub develops. Also, the similarity between Q. microphylla and Q. frutex, mainly in geographically close populations, can still generate taxonomic confusion. All these variations generate uncertainty about the existence of other unrecognized taxa within the analyzed populations. Therefore, evaluating the different species hypotheses with molecular data can greatly contribute to clarify the identity of these variants.
Data availability
Original data used for this study are available upon request to the corresponding author.
References
Adams DC, Collyer M, Kaliontzopoulou A, Sherratt E (2016) Geomorph: software for geometric morphometric analyses
Aiello-Lammens ME, Boria RA, Radosavljevic A et al (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545. https://doi.org/10.1111/ecog.01132
Albarrán-Lara AL, Mendoza-Cuenca L, Valencia-Avalos S et al (2010) Leaf fluctuating asymmetry increases with hybridization and introgression between Quercus magnoliifolia and Quercus resinosa (Fagaceae) through an altitudinal gradient in Mexico. Int J Plant Sci 171:310–322. https://doi.org/10.1086/650317
Aykut Y, Emel U, Tekin BM (2017) Morphological variability of evergreen oaks (Quercus) in Turkey. Bangladesh J Plant Taxon 24(1):39–47. https://doi.org/10.3329/bjpt.v24i1.33004
Bartholomew B, Almeda F (2023) Nomenclator botanicus of Fagaceae in Latin America. Phytotaxa 580:1–97
Bickford D, Lohman DJ, Sodhi NS et al (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155. https://doi.org/10.1016/j.tree.2006.11.004
Borazan A, Babaç MT (2003) Morphometric leaf variation in oaks (Quercus) of Bolu, Turkey. Ann Bot Fenn 40:233–242
Cannon CH, Petit RJ (2020) The oak syngameon: more than the sum of its parts. New Phytol 226:978–983. https://doi.org/10.1111/nph.16091
Cavender-Bares J (2019) Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol 221:669–692. https://doi.org/10.1111/nph.15450
Chitwood DH, Sinha NR (2016) Evolutionary and environmental forces sculpting leaf development. Curr Biol 26:R297–R306. https://doi.org/10.1016/j.cub.2016.02.033
De Heredia UL, Duro-García MJ, Soto Á (2018) Leaf morphology of progenies in Q. suber, Q. ilex, and their hybrids using multivariate and geometric morphometric analysis. Iforest 11:90–98. https://doi.org/10.3832/ifor2577-010
Denk T, Grimm GW, Manos PS et al (2017) An updated infrageneric classification of the oaks: review of previous taxonomic schemes and synthesis of evolutionary patterns. In: Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D (eds) Oaks physiological ecology. Exploring the functional diversity of genus Quercus L. Springer International Publishing, Cham, pp 13–38
Di Pietro R, Conte AL, Di Marzio P et al (2020) A multivariate morphometric analysis of diagnostic traits in southern Italy and Sicily pubescent oaks. Folia Geobot 55:163–183. https://doi.org/10.1007/s12224-020-09378-0
Dumolin-Lapégue S, Kremer A, Petit RJ (1999) Are chloroplast and mitochondrial DNA variation species independent in oaks? Evolution (N Y) 53:1406–1413. https://doi.org/10.1111/j.1558-5646.1999.tb05405.x
Elith J, Phillips SJ, Hastie T et al (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57. https://doi.org/10.1111/j.1472-4642.2010.00725.x
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Fortini P, Antonecchia G, Di Marzio P et al (2015) Role of micromorphological leaf traits and molecular data in taxonomy of three sympatric white oak species and their hybrids (Quercus L.). Plant Biosyst 149:546–558
Galicia L, Potvin C, Messier C (2015) Maintaining the high diversity of pine and oak species in mexican temperate forests: a new management approach combining functional zoning and ecosystem adaptability. Can J for Res 45:1358–1368. https://doi.org/10.1139/cjfr-2014-0561
González Villarreal LM (1986) Contribución al conocimiento del género Quercus (Fagaceae) en el estado de Jalisco. Instituto de Botánica. Universidad de Guadalajara, Guadalajara
González-Elizondo SM, González-Elizondo M, Cortés-Ortiz A (1993) Vegetación de la reserva de la biosfera “La Michilía”, Durango, México. Acta Bot Mex. https://doi.org/10.21829/abm22.1993.668
González-Rodríguez A, Bain JF, Golden JL, Oyama K (2004) Chloroplast DNA variation in the Quercus affinis-Q. laurina complex in Mexico: geographical structure and associations with nuclear and morphological variation. Mol Ecol 13:3467–3476. https://doi.org/10.1111/j.1365-294X.2004.02344.x
González VLM (1986) Contribución al conocimiento del género Quercus (Fagaceae) en el estado de Jalisco. Universidad de Guadalajara, México
Hardin JW (1975) Hybridization and introgression in Quercus alba. J Arnold Arboretum 56:336–363
Hipp AL, Manos PS, González-Rodríguez A et al (2018) Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytol 217:439–452. https://doi.org/10.1111/nph.14773
Hipp AL, Manos PS, Hahn M et al (2020) Genomic landscape of the global oak phylogeny. New Phytol 226:1198–1212. https://doi.org/10.1111/nph.16162
Jensen RJ (1990) Detecting shape variation in oak leaf morphology: a comparison of rotational-fit methods. Am J Bot 77:1279–1293. https://doi.org/10.1002/j.1537-2197.1990.tb11380.x
Jones JH (1986) Evolution of the Fagaceae: the implications of foliar features. Ann Missouri Bot Garden 73:228–275
Leroy T, Roux C, Villate L et al (2017) Extensive recent secondary contacts between four European white oak species. New Phytol 214:865–878. https://doi.org/10.1111/nph.14413
Lobo JM, Jiménez-valverde A, Real R (2008) AUC: a misleading measure of the performance of predictive distribution models. Global Ecol Biogeogr 17:145–151
López-Caamal A, Ruiz-Amaro LDC, Zepeda-Rodríguez A et al (2017) Micromorphological character expression of the hybrid Quercus × dysophylla and its parental species (Q. crassifolia and Q. crassipes). Bot Sci 95:375–389. https://doi.org/10.17129/botsci.875
Martínez M (1977) Los encinos de México. Anales del Instituto de Biología: México Comisión Forestal. pp 230
Martinez-Meyer E (2005) Climate change and biodiversity: some considerations in forecasting shifts in species’ potential distributions. Biodiv Inf. https://doi.org/10.17161/bi.v2i0.8
McCauley RA, Cortés-Palomec AC, Oyama K (2019) Species diversification in a lineage of Mexican red oak (Quercus section Lobatae subsection Racemiflorae) the interplay between distance, habitat, and hybridization. Tree Genet Genomes. https://doi.org/10.1007/s11295-019-1333-x
McVaugh R (1974) Flora Novo-Galiciana: Fagaceae. University Herbarium, University of Michigan, Ann Arbor
Morales-Saldaña S, Valencia-Ávalos S, Oyama K et al (2022) Even more oak species in Mexico? Genetic structure and morphological differentiation support the presence of at least two specific entities within Quercus laeta. J Syst Evol. https://doi.org/10.1111/jse.12818
Muller CH (1944) Fagaceae. J Arnold Arboretum 25:437–450
Nixon KC (1993) Infrageneric classification of Quercus (Fagaceae) and typification of sectional names. Ann Sci For 50:25–34
Nosil P, Feder JL (2012) Genomic divergence during speciation: causes and consequences. Philos Trans Royal Soc B: Biol Sci 367:332–342. https://doi.org/10.1098/rstb.2011.0263
Olson DM, Dinerstein E, Wikramanayake ED et al (2001) Terrestrial ecoregions of the world: a new map of life on earth: a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 51:933–938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Ortego J, Noguerales V, Gugger PF, Sork VL (2015) Evolutionary and demographic history of the Californian scrub white oak species complex: an integrative approach. Mol Ecol 24:6188–6208. https://doi.org/10.1111/mec.13457
Pearson RG, Raxworthy CJ, Nakamura M, Townsend Peterson A (2007) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34:102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x
Pérez-Pedraza A, Rodríguez-Correa H, Valencia-Ávalos S et al (2021) Effect of hybridization on the morphological differentiation of the red oaks Quercus acutifolia and Quercus grahamii (Fagaceae). Plant Syst Evol 307:1–14. https://doi.org/10.1007/s00606-021-01757-0
Peterson AT, Nakazawa Y (2008) Environmental data sets matter in ecological niche modelling: An example with Solenopsis invicta and Solenopsis richteri. Global Ecol Biogeogr 17:135–144. https://doi.org/10.1111/j.1466-8238.2007.00347.x
Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Modell 213:63–72. https://doi.org/10.1016/j.ecolmodel.2007.11.008
Peterson AT, Soberón J, Pearson RG et al (2011) Ecological niches and geographic distributions: monographs in population biology. Princeton University Press, Princeton
Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175. https://doi.org/10.1111/j.0906-7590.2008.5203.x
Phillips SB, Aneja VP, Kang D, Arya SP (2006) Modelling and analysis of the atmospheric nitrogen deposition in North Carolina. Int J Global Environ Issues 6:231–252
Pinheiro F, Dantas-Queiroz MV, Palma-Silva C (2018) Plant species complexes as models to understand speciation and evolution: a review of South American studies. CRC Crit Rev Plant Sci 37:54–80. https://doi.org/10.1080/07352689.2018.1471565
Proietti E, Filesi L, Di Marzio P et al (2021) Morphology, geometric morphometrics, and taxonomy in relict deciduous oaks woods in northern Italy. Rendiconti Lincei 32:549–564. https://doi.org/10.1007/s12210-021-01001-4
QGIS Development Team. 2019. QGIS Geographic information system. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
R Core TeamR Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rodríguez-Gómez F, Oyama K, Ochoa-Orozco M et al (2018) Phylogeography and climate-associated morphological variation in the endemic white oak Quercus deserticola (Fagaceae) along the Trans-Mexican Volcanic Belt. Botany 96:121–131. https://doi.org/10.1139/cjb-2017-0116
Rohlf FJ (2006) tpsDig 2.10. Department of Ecology and Evolution, State University of New York, Stony Brook, NY
Romero RS, Rojas ZEC, Aguilar EML (2002) El Género Quercus (Fagaceae) en el Estado de México. Ann Mo Bo Gard 89(4):551–593. https://doi.org/10.2307/3298595
Romero Rangel S (2006) Revisión taxonómica del complejo Acutifoliae de Quercus (Fagaceae) con énfasis en su representación en México. Acta Bot Mex 76:1–45
Romero Rangel S, Rojas Zenteno EC, Enríquez A, de Lourdes M (2002) El Género Quercus ( Fagaceae ) en el Estado de México. Ann Missouri Bot Garden 89:551–593
Romero Rangel S, Rojas Zenteno EC, Rubio Licona LE (2014) Fagaceae. Flora Del bajío y de Regiones Adyacentes 42: 87–110. https://doi.org/10.1016/0034-6667(84)90063-0
Sabás-Rosales JL, Siqueiros-Delgado ME, Valencia-Ávalos S, Enríquez-Enríquez ED (2017) Reconocimiento taxonómico de seis especies arbustivas de encinos (Quercus secc. Quercus: Fagaceae). Polibotanica. https://doi.org/10.18387/polibotanica.44.2
Scareli-Santos C, Sánchez-Mondragón ML, González-Rodríguez A, Oyama K (2013) Micromorphology of Mexican Oaks (Quercus: Fagaceae). Acta Bot Mex 104:31–52
Schlager S (2017) Morpho and Rvcg-Shape Analysis in R: R-Packages for geometric morphometrics, shape analysis and surface manipulations. Statistical shape and deformation analysis. Elsevier, Amsterdam, pp 217–256
Sork VL, Riordan E, Gugger PF et al (2016) Phylogeny and introgression of California scrub white oaks (Quercus section Quercus). Int Oaks 27:1–14
Trelease W (1924) The american oaks. Mem Natl Acad Sci 20:1–255
Tschan GF, Denk T (2012) Trichome types, foliar indumentum and epicuticular wax in the Mediterranean gall oaks, Quercus subsection Galliferae (Fagaceae): Implications for taxonomy, ecology and evolution. Bot J Linnean Soc 169:611–644. https://doi.org/10.1111/j.1095-8339.2012.01233.x
Tucker JM (1974) Patterns of parallel evolution of leaf form in new world oaks. Taxon 23:129–154. https://doi.org/10.2307/1218095
Valencia-A S (2004) Diversidad del género Quercus (Fagaceae) en México. Bot Sci 75:33–53. https://doi.org/10.17129/botsci.1692
Valencia-A S (2020) Species delimitation in the genus Quercus (Fagaceae). Bot Sci 99:1–12. https://doi.org/10.17129/botsci.2658
Valencia-A S, Sabas Rosales JL, Soto Arellano OJ (2016) A new species of Quercus, section Lobatae (Fagaceae) from the Sierra Madre oriental, Mexico. Phytotaxa 269:120–126. https://doi.org/10.11646/phytotaxa.269.2.5
Valencia-A S, Flores-Franco G, Jiménez-Ramírez J, Mora-Jarvio M (2017) Distribution and diversity of Fagaceae in Hidalgo, Mexico. Bot Sci 95:660–721. https://doi.org/10.17129/botsci.1020
Valencia-Cuevas L, Mussali-Galante P, Piñero D et al (2015) Hybridization of Quercus castanea (Fagaceae) across a red oak species gradient in Mexico. Plant Syst Evol 301:1085–1097. https://doi.org/10.1007/s00606-014-1151-4
Vázquez-Villagrán L (2000) Flora del valle de Tehuacán-Cuicatlán. Fagaceae. Universidad Nacional Autónoma De México Instituto De Biología 28:1–39
Villareal JA, Encina JA, Carranza MA (2008) Los encinos (Quercus: Fagaceae) de Coahuila, México. J Bot Res Inst Tex 2:1235–1278. https://doi.org/10.1111/conl.12288.This
Viscosi V (2015) Geometric morphometrics and leaf phenotypic plasticity: assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot J Linnean Soc 179:335–348. https://doi.org/10.1111/boj.12323
Viscosi V, Fortini P, Slice DE et al (2009a) Geometric morphometric analyses of leaf variation in four oak species of the subgenus Quercus (Fagaceae). Plant Biosyst 143:575–587. https://doi.org/10.1080/11263500902775277
Viscosi V, Lepais O, Gerber S, Fortini P (2009b) Leaf morphological analyses in four European oak species (Quercus) and their hybrids: a comparison of traditional and geometric morphometric methods. Plant Biosyst 143:564–574. https://doi.org/10.1080/11263500902723129
Viscosi V, Loy A, Fortini P (2010) Geometric morphometric analysis as a tool to explore covariation between shape and other quantitative leaf traits in European white oaks. Tools for identifying Biodiversity: Progress and Problems 257–261
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution (N Y) 62:2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Warren DL, Glor RE, Turelli M (2010) ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33:607–611. https://doi.org/10.1111/j.1600-0587.2009.06142.x
Warren DL, Matzke NJ, Cardillo M et al (2021) ENMTools 1.0: an R package for comparative ecological biogeography. Ecography 44:504–511. https://doi.org/10.1111/ecog.05485
Whittemore AT, Schaal BA (1991) Interspecific gene flow in sympatric oaks. Proc Natl Acad Sci USA 88:2540–2544. https://doi.org/10.1073/pnas.88.6.2540
Wu Y, Hipp AL, Fargo G et al (2023) Improving species delimitation for effective conservation: a case study in the endemic maple-leaf oak (Quercus acerifolia). New Phytol 238:1278–1293. https://doi.org/10.1111/nph.18777
Yang K, Wu J, Li X et al (2022) Intraspecific leaf morphological variation in Quercus dentata Thunb.: a comparison of traditional and geometric morphometric methods, a pilot study. J For Res (Harbin). https://doi.org/10.1007/s11676-022-01452-x
Acknowledgements
We thank Gonzalo Contreras, Goretty Mendoza, Ricardo Gaytán, Javier Piña and Douglas Castillejo for their collaboration in the field work. Gonzalo Contreras helped us with the statistical analysis. We also thank to MSci Violeta Patiño Conde for her assistance in obtaining microscope images. This work constitutes a partial fulfillment of the Posgrado en Ciencias Biológicas of the National Autonomous University of Mexico (UNAM). Oscar De Luna, thanks Posgrado en Ciencias Biológicas-UNAM and the Consejo Nacional de Ciencia y Tecnología (CONACYT) for the PhD scholarship and financial support to develop graduate studies at UNAM (scholarship/CVU:717866/742699). The authors are grateful for funding from the Dirección General de Asuntos del Personal Académico (DGAPA)-Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT IN210020). We are also grateful for additional funding received from the Association de l'Arboretum des Pouyouleix through Béatrice Chassé.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Luna-Bonilla, O.Á., Valencia-Á, S., Ibarra-Manríquez, G. et al. Leaf morphometric analysis and potential distribution modelling contribute to taxonomic differentiation in the Quercus microphylla complex. J Plant Res 137, 3–19 (2024). https://doi.org/10.1007/s10265-023-01495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-023-01495-z