Abstract
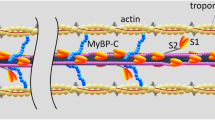
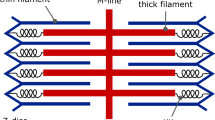
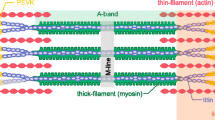
Although recent research emphasises the possible role of titin in skeletal muscle force enhancement, this property is commonly ignored in current computational models. This work presents the first biophysically based continuum-mechanical model of skeletal muscle that considers, in addition to actin–myosin interactions, force enhancement based on actin–titin interactions. During activation, titin attaches to actin filaments, which results in a significant reduction in titin’s free molecular spring length and therefore results in increased titin forces during a subsequent stretch. The mechanical behaviour of titin is included on the microscopic half-sarcomere level of a multi-scale chemo-electro-mechanical muscle model, which is based on the classic sliding-filament and cross-bridge theories. In addition to titin stress contributions in the muscle fibre direction, the continuum-mechanical constitutive relation accounts for geometrically motivated, titin-induced stresses acting in the muscle’s cross-fibre directions. Representative simulations of active stretches under maximal and submaximal activation levels predict realistic magnitudes of force enhancement in fibre direction. For example, stretching the model by 20 % from optimal length increased the isometric force at the target length by about 30 %. Predicted titin-induced stresses in the muscle’s cross-fibre directions are rather insignificant. Including the presented development in future continuum-mechanical models of muscle function in dynamic situations will lead to more accurate model predictions during and after lengthening contractions.








Similar content being viewed by others
References
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117(1):77–86
Baylor S, Hollingworth S (2003) Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol 551(1):125–138
Baylor SM, Hollingworth S (1998) Model of sarcomeric Ca\(^{2+}\) movements, including ATP Ca\(^{2+}\) binding and diffusion, during activation of frog skeletal muscle. J General Physiol 112(3):297–316. doi:10.1085/jgp.112.3.297
Bianco P, Nagy A, Kengyel A, Szatmári D, Mártonfalvi Z, Huber T, Kellermayer MS (2007) Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J 93(6):2102–2109
Bianco P, Mártonfalvi Z, Naftz K, Kőszegi D, Kellermayer M (2015) Titin domains progressively unfolded by force are homogenously distributed along the molecule. Biophys J 109(2):340–345
Biewener A, Baudinette R (1995) In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (macropus eugenii). J Exp Biol 198(9):1829–1841
Blemker SS, Delp SL (2005) Three-dimensional representation of complex muscle architectures and geometries. Ann Biomed Eng 33(5):661–673
Blemker SS, Pinsky PM, Delp SL (2005) A 3D model of muscle reveals the causes of nonuniform strains in the biceps brachii. J Biomech 38(4):657–665. doi:10.1016/j.jbiomech.2004.04.009
Blickhan R, Seyfarth A, Geyer H, Grimmer S, Wagner H, Günther M (2007) Intelligence by mechanics. Philos Trans R Soc Lond A Math Phys Eng Sci 365(1850):199–220
Bradley CP, Bowery A, Britten R, Budelmann V, Camara O, Christie R, Cookson A, Frangi AF, Gamage T, Heidlauf T, Krittian S, Ladd D, Little C, Mithraratne K, Nash M, Nickerson D, Nielsen P, NordbøO Omholt S, Pashaei A, Paterson D, Rajagopal V, Reeve A, Röhrle O, Safaei S, Sebastián R, Steghöfer M, Wu T, Yu T, Zhang H, Hunter PJ (2011) OpenCMISS: A multi-physics and multi-scale computational infrastructure for the VPH/Physiome project. Prog Biophys Mol Biol 107:32–47. doi:10.1016/j.pbiomolbio.2011.06.015
Campbell SG, Campbell KS (2011) Mechanisms of residual force enhancement in skeletal muscle: insights from experiments and mathematical models. Biophys Rev 3(4):199–207
Curtin NA, Davies RE (1975) Very high tension with very little ATP breakdown by active skeletal muscle. J Mechanochem Cell Motil 3(2):147–154
Edman K (1979) The velocity of unloaded shortening and its relation to sarcomere length and isometric force on vertebrate muscle fibres. J Physiol 291:143–159
Edman K (2012) Residual force enhancement after stretch in striated muscle. A consequence of increased myofilament overlap? J Physiol 590(6):1339–1345
Edman KA, Elzinga G, Noble MI (1978) Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol 281(1):139–155
Elliott G, Lowy J, Worthington C (1963) An X-ray and light-diffraction study of the filament lattice of striated muscle in the living state and in rigor. J Mol Biol 6(4):295–305
Gillies AR, Lieber RL (2011) Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44(3):318–331
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192
Günther M, Röhrle O, Haeufle DF, Schmitt S (2012) Spreading out muscle mass within a hill-type model: a computer simulation study. Comput Math Methods Med. doi:10.1155/2012/848630
Häufle D, Günther M, Wunner G, Schmitt S (2014) Quantifying control effort of biological and technical movements: an information-entropy-based approach. Phys Rev E 89(1):012,716
Heidlauf T, Röhrle O (2013) Modeling the chemoelectromechanical behavior of skeletal muscle using the parallel open-source software library OpenCMISS. Comput Math Methods Med 2013:1–14. doi:10.1155/2013/517287
Heidlauf T, Röhrle O (2014) A multiscale chemo-electro-mechanical skeletal muscle model to analyze muscle contraction and force generation for different muscle fiber arrangements. Front Physiol 5(498):1–14. doi:10.3389/fphys.2014.00498
Herzog W, Leonard T (2002) Force enhancement following stretching of skeletal muscle: a new mechanism. J Exp Biol 205(9):1275–1283
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B 126(843):136–195
Holzapfel GA (2000) Nonlinear solid mechanics. Wiley, Chichester
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction: interference microscopy of living muscle fibres. Nature 173:971–973
Huxley H, Hanson J (1954) Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173:973–976
Joumaa V, Rassier DE, Leonard TR, Herzog W (2007) Passive force enhancement in single myofibrils. Pflügers Arch Eur J Physiol 455(2):367–371
Joumaa V, Leonard TR, Herzog W (2008) Residual force enhancement in myofibrils and sarcomeres. Proc R Soc Lond B Biol Sci 275(1641):1411–1419
Keener J, Sneyd J (2009) Mathematical physiology I: cellular physiology, vol 1, 2nd edn. Springer, Berin
Kellermayer MS, Granzier HL (1996) Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Lett 380(3):281–286
Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA (2001) Interaction between PEVK-titin and actin filaments origin of a viscous force component in cardiac myofibrils. Circ Res 89(10):874–881
Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Nat Acad Sci 100(23):13,716–13,721
Lee EJ, Herzog W (2008) Residual force enhancement exceeds the isometric force at optimal sarcomere length for optimized stretch conditions. J Appl Physiol 105(2):457–462
Lee HD, Herzog W (2002) Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol 545(1):321–330
Lemos RR, Epstein M, Herzog W, Wyvill B (2001) Realistic skeletal muscle deformation using finite element analysis. XIV Brazilian symposium on computer graphics and image processing, pp 192–199
Leonard TR, Herzog W (2010) Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am J Physiol Cell Physiol 299(1):C14–C20
Linke WA, Krüger M (2010) The giant protein titin as an integrator of myocyte signaling pathways. Physiology 25(3):186–198
Linke WA, Ivemeyer M, Mundel P, Stockmeier MR, Kolmerer B (1998a) Nature of PEVK-titin elasticity in skeletal muscle. Proc Nat Acad Sci 95(14):8052–8057
Linke WA, Stockmeier MR, Ivemeyer M, Hosser H, Mundel P (1998b) Characterizing titin’s I-band Ig domain region as an entropic spring. J Cell Sci 111(11):1567–1574
Maas H, Baan GC, Huijing PA (2001) Intermuscular interaction via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J Biomech 34(7):927–940
Meijer K (2002) History dependence of force production in submaximal stimulated rat medial gastrocnemius muscle. J Electromyogr Kinesiol 12(6):463–470
Mordhorst M, Heidlauf T, Röhrle O (2015) Predicting electromyographic signals under realistic conditions using a multiscale chemo-electro-mechanical finite element model. Interf Focus 5. doi:10.1098/rsfs.2014.0076
Morgan D (1990) New insights into the behavior of muscle during active lengthening. Biophys J 57(2):209–221
Morgan D, Mochon S, Julian F (1982) A quantitative model of intersarcomere dynamics during fixed-end contractions of single frog muscle fibers. Biophys J 39(2):189–196
Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U (2000) Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol 522(3):503–513
Müller R, Siebert T, Blickhan R et al (2012) Muscle preactivation control: simulation of ankle joint adjustments at touchdown during running on uneven ground. J Appl Biomech 28(6):718–725
Nagy JG, Palmer K, Perrone L (2004) Iterative methods for image deblurring: a matlab object-oriented approach. Numer Algor 36:73–93
Niederländer N, Raynaud F, Astier C, Chaussepied P (2004) Regulation of the actin–myosin interaction by titin. Eur J Biochem 271(22):4572–4581
Nishikawa KC, Monroy JA, Uyeno TE, Yeo SH, Pai DK, Lindstedt SL (2012) Is titin a ’winding filament’? A new twist on muscle contraction. Proc R Soc Lond B Biol Sci 279(1730):981–990
Noble MI (1992) Enhancement of mechanical performance of striated muscle by stretch during contraction. Exp Physiol 77(4):539–552
Peterson DR, Rassier DE, Herzog W (2004) Force enhancement in single skeletal muscle fibres on the ascending limb of the force–length relationship. J Exp Biol 207(16):2787–2791
Powers K, Schappacher-Tilp G, Jinha A, Leonard T, Nishikawa K, Herzog W (2014) Titin force is enhanced in actively stretched skeletal muscle. J Exp Biol 217(20):3629–3636
Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA (2005) Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J General Physiol 126(5):461–480
Razumova MV, Bukatina AE, Campbell KB (1999) Stiffness-distortion sarcomere model for muscle simulation. J Appl Physiol 87(5):1861–1876
Rode C, Siebert T, Blickhan R (2009) Titin-induced force enhancement and force depression: a ‘sticky-spring’ mechanism in muscle contractions? J Theor Biol 259(2):350–360. doi:10.1016/j.jtbi.2009.03.015
Röhrle O, Pullan AJ (2007) Three-dimensional finite element modelling of muscle forces during mastication. J Biomech 40(15):3363–3372
Röhrle O, Davidson JB, Pullan AJ (2008) Bridging scales: a three-dimensional electromechanical finite element model of skeletal muscle. SIAM J Sci Comput 30(6):2882–2904. doi:10.1137/070691504
Schappacher-Tilp G, Leonard T, Desch G, Herzog W (2015) A novel three-filament model of force generation in eccentric contraction of skeletal muscles. PLOS ONE 10(3):e0117634
Shorten PR, O’Callaghan P, Davidson JB, Soboleva TK (2007) A mathematical model of fatigue in skeletal muscle force contraction. J Muscle Res Cell Motil 28(6):293–313. doi:10.1007/2Fs10974-007-9125-6
Siebert T, Rode C (2014) Computational modeling of muscle biomechanics. In: Jin Z(ed) Computational modelling of biomechanics and biotribology in the musculoskeletal system: biomaterials and tissues, 1st edn. Woodhead Publishing, Elsevier, Amsterdam, pp 173–204
Siebert T, Günther M, Blickhan R (2012) A 3D-geometric model for the deformation of a transversally loaded muscle. J Theor Biol 298:116–121
Siebert T, Leichsenring K, Rode C, Wick C, Stutzig N, Schubert H, Blickhan R, Böl M (2015) Three-dimensional muscle architecture and comprehensive dynamic properties of rabbit gastrocnemius, plantaris and soleus: Input for simulation studies. PLOS ONE 10(6):e0130985
Till O, Siebert T, Rode C, Blickhan R (2008) Characterization of isovelocity extension of activated muscle: a Hill-type model for eccentric contractions and a method for parameter determination. J Theor Biol 255(2):176–187. doi:10.1016/j.jtbi.2008.08.009
Till O, Siebert T, Blickhan R (2010) A mechanism accounting for independence on starting length of tension increase in ramp stretches of active skeletal muscle at short half-sarcomere lengths. J Theor Biol 266(1):117–123
Tskhovrebova L, Trinick J (2003) Titin: properties and family relationships. Nat Rev Mol Cell Biol 4(9):679–689
Usyk T, Mazhari R, McCulloch A (2000) Effect of laminar orthotropic myofiber architecture on regional stress and strain in the canine left ventricle. J Elast Phys Sci Solids 61(1–3):143–164
Van Loocke M, Lyons CG, Simms CK (2006) A validated model of passive muscle in compression. J Biomech 39(16):2999–3009. doi:10.1016/j.jbiomech.2005.10.016
Walcott S, Herzog W (2008) Modeling residual force enhancement with generic cross-bridge models. Math Biosci 216(2):172–186
Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED, Ypey DL (1999) Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J 28(4):317–329. doi:10.1007/s002490050214
Yucesoy CA, Koopman BH, Grootenboer HJ, Huijing PA (2007) Finite element modeling of aponeurotomy: altered intramuscular myofascial force transmission yields complex sarcomere length distributions determining acute effects. Biomech Model Mechanobiol 6(4):227–243
Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M (2006) Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature 439(7073):229–233
Zuurbier CJ, Huijing PA (1992) Influence of muscle geometry on shortening speed of fibre, aponeurosis and muscle. J Biomech 25(9):1017–1026
Acknowledgments
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013) / ERC Grant Agreement n. 306757. Moreover, the authors T. H., E. A., C. B., and O. R. would like to thank the German Research Foundation (DFG) for the financial support of the project within the Cluster of Excellence in Simulation Technology (EXC 310/1) at the University of Stuttgart.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidlauf, T., Klotz, T., Rode, C. et al. A multi-scale continuum model of skeletal muscle mechanics predicting force enhancement based on actin–titin interaction. Biomech Model Mechanobiol 15, 1423–1437 (2016). https://doi.org/10.1007/s10237-016-0772-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0772-7