Abstract
This study characterized metal contamination in the Blesbokspruit River waters, near the coal-mining town of Emalahleni in Mpumalanga, South Africa, using enrichment factors (EFs) and contamination factors (CFs). We investigated the potential of Fe and Al ‘dilution factors’ (stream water divided by overbank sediment concentrations) under base flow hydrological conditions to detect AMD-related trace metal contamination in the study site. Waters of the Blesbokspruit River were characterized by acidic (< 3) to near neutral (≈7) pH, high EC (up to 2240 µS/cm), high sulfate (up to 1530 mg/L), ultra-high degrees of Fe contamination and minor to moderately severe enrichment of trace metals, all of which strongly indicate that the river is affected by AMD. Fe and Al dilution factors showed moderate to strong positive correlations with dissolved trace metals (Co, Ni, Zn, Pb, Cr, and Cd), but not with Cu, which is likely more associated with kaolinite in the study area. In addition, correlation analysis showed that dilution factors were better for detecting aqueous Co and Cd, and comparable for detecting aqueous Ni, relative to stream pH and aqueous Fe and Al. Dilution factors have an advantage over stream pH and aqueous Al and Fe because, once calculated, they can be used to monitor AMD-related trace metal contamination in streams during dry seasons by using only Al and Fe content in sediments. This can be beneficial when preservation methods or storage necessary for water samples are not available. However, the robustness of dilution factors during wet seasons requires further investigation.
摘要
本研究使用富集因子(EFS)和污染因子(CFS)对位于南非Mpumalanga省的Emalahleni煤矿小镇附近的Blesbokspruit水域的金属污染进行了表征。我们研究了Fe和Al‘稀释系数’(以河岸泥沙浓度划分的溪流)在基流水文条件下的潜在性,以检测研究区AMD相关的微量金属污染。Blesbokspruit水体的PH值呈酸性(<3)至近中性(≈7)、高EC值(达2240µS/cm),高硫酸盐含量(达1530 mg/L)、极高的铁污染以及轻到中重度的微量金属富集。这些特征强烈表明河流受到了AMD的影响。Fe、Al稀释系数与溶解的微量金属(Co、Ni、Zn、Pb、Cr、Cd)呈中到强的正相关,而与Cu则无明显的正相关关系,Cu可能与研究区高岭石的相关性更强。此外,相关分析表明,相对于溪流pH值以及水中的Fe、Al而言,稀释系数对Co和Cd的检测效果更好,对水中Ni的测定相当。稀释系数比溪流pH值、水中的Al和Fe更有优势,因为一旦计算出来,它们可以仅利用沉积物中Al和Fe的含量来监测旱季溪流中与AMD有关的微量金属污染。这对于不能保存或储存的水样是有益的。然而,稀释系数在雨季的稳定性还需要进一步的研究。
Zusammenfassung
In dieser Studie wurde die Metallkontamination im Wasser des Blesbokspruit-Flusses in der Nähe der Bergbaustadt Emalahleni in Mpumalanga, Südafrika, anhand von Anreicherungsfaktoren (EF) und Kontaminationsfaktoren (CF) charakterisiert. Wir untersuchten das Potenzial von Fe- und Al-Verdünnungsfaktoren (Flusswasser geteilt durch Konzentrationen in den Auesedimenten) bei Niedrigwasser (Basisabfluss), um AMD-bedingte Spurenmetallkontaminationen im Untersuchungsgebiet zu erkennen. Das Wasser des Blesbokspruit-Flusses zeichnete sich durch einen sauren (< 3) bis nahezu neutralen (≈7) pH-Wert, einen hohen EC-Wert (bis zu 2240 µS/cm), einen hohen Sulfatgehalt (bis zu 1530 mg/L), einen sehr hohen Grad an Fe-Kontamination und eine geringfügige bis mäßig starke Anreicherung von Spurenmetallen aus, die alle stark darauf hindeuten, dass der Fluss von AMD betroffen ist. Die Fe- und Al-Verdünnungsfaktoren wiesen mäßige bis starke positive Korrelationen mit gelösten Spurenmetallen (Co, Ni, Zn, Pb, Cr und Cd) auf, nicht jedoch mit Cu, das im Untersuchungsgebiet wahrscheinlich eher mit Kaolinit assoziiert ist. Darüber hinaus zeigten Korrelationsanalysen, dass die Verdünnungsfaktoren für den Nachweis von wässrigem Co und Cd besser und für den Nachweis von wässrigem Ni vergleichbar sind, im Vergleich zum pH-Wert des Flusses und wässrigem Fe und Al. Die berechneten Verdünnungsfaktoren haben den Vorteil gegenüber dem pH-Wert des Flusses und den Konzentrationen von Al und Fe im Wasser, dass sie in der Trockenzeit für das Monitoring von AMD-bedingten Spurenmetallen im Flusswasser benutzt werden können, allein basierend auf den Fe- und Al-Sedimentkonzentrationen. Dies kann von Vorteil sein, wenn die für Wasserproben erforderlichen Konservierungsmethoden oder die Lagerung nicht möglich sind. Die Robustheit der Verdünnungsfaktoren in nassen Jahreszeiten muss jedoch weiter untersucht werden.
Resumen
Este estudio caracterizó la contaminación por metales en las aguas del río Blesbokspruit, cerca de la ciudad minera de carbón de Emalahleni en Mpumalanga, Sudáfrica, utilizando factores de enriquecimiento (EF) y factores de contaminación (CF). En este trabajo se investiga el potencial de los 'factores de dilución' de Fe y Al (agua del arroyo dividida por concentraciones de sedimentos fuera de la orilla) bajo condiciones hidrológicas de flujo base para detectar la contaminación por metales traza relacionada con AMD en la zona de estudio. Las aguas del río Blesbokspruit se caracterizaron por tener un pH ácido (< 3) a casi neutro (≈7), alta conductividad eléctrica (CE) (hasta 2240 µS/cm), alta concentración de sulfato (hasta 1530 mg/L), un grado extremo de contaminación de Fe y un enriquecimiento en metales traza de leves a moderadamente severos, lo que indica claramente que el río está afectado por AMD. Los factores de dilución de Fe y Al mostraron correlaciones positivas moderadas a fuertes con metales traza disueltos (Co, Ni, Zn, Pb, Cr y Cd), pero no con Cu, que probablemente esté más asociado con caolinita en el área de estudio. Además, el análisis de correlación mostró que los factores de dilución eran mejores para detectar Co y Cd disueltos, y comparables para detectar Ni disuelto, en relación con el pH del arroyo y Fe y Al disueltos. Los factores de dilución tienen una ventaja sobre el pH y la concentración disuelta de Fe y Al porque, una vez calculados, pueden utilizarse para monitorear la contaminación por metales traza relacionada con AMD en los arroyos durante las estaciones secas utilizando solo el contenido de Al y Fe en los sedimentos. Esto puede ser beneficioso cuando no están disponibles los métodos de preservación o el almacenamiento necesario para las muestras de agua. Sin embargo, la robustez de los factores de dilución durante las estaciones húmedas requiere una investigación adicional.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid mine drainage (AMD) is a serious environmental problem that affects mining areas and its surroundings around the world (Abrahams and Carranza 2023; Adeniyi et al. 2022; Cánovas et al. 2022; Kumar et al. 2023; Nascimento et al. 2023; Ojonimi et al. 2021). It is an acidic effluent generated as a result of metal-sulphide oxidation in the presence of air and water and it is rich in sulfates and metals (Skousen 2014; Skousen et al. 1999). Acidic conditions can favor the leaching of trace metals from surrounding host rocks and waste materials into surface waters (Masindi et al. 2017; Skousen et al. 2019). Elevated trace metal concentrations are among the biggest concerns associated with AMD. Unlike many organic compounds, trace metals are non-biodegradable and continue to accumulate within the environment over time (Manahan 1991; Uysal et al. 2009). Several trace metals, such as Cu, Zn, and Co, are considered crucial for the maintenance of health in biological systems (Ali et al. 2019), but can become toxic to the environment and humans at elevated concentrations (Alomary and Belhadj 2007). In addition, trace metals such as Pb and Cd are toxic at even very low levels of exposure (Tchounwou et al. 2012). Thus, the detection and monitoring of trace metal concentrations within the environment is crucial to mitigate the associated health risks (Bakirdere and Yaman 2008).
Streambed and overbank sediments along streams and rivers draining mines can act as important sinks and secondary sources of trace metals (Galán et al. 2003; Kos et al. 2022; Schulz-Zunkel and Krueger 2009). This is largely due to the presence of secondary oxides and oxyhydroxides (Campaner et al. 2014; Parker et al. 2007; Schaider et al. 2014; Zhao et al. 2012), which are known to concentrate trace metals in soils and sediments because of their high specific surface areas, high cation exchange capacities and affinity for forming colloids and coatings on other minerals (Sparks 2002; Wilkin 2008). Thus, they play a crucial role in regulating dissolved trace metal concentrations along streams and rivers (Webster et al. 1998).
Conventional geochemical techniques for detecting and monitoring trace metal contamination in the environment typically require: extensive field sampling, thorough sample preservation, the use of strong acid digestions, and expensive (priced per element analyzed) laboratory analysis, all of which can be very time-consuming (Pandit et al. 2010). As a result, conventional geochemical techniques can often be inefficient and expensive when performed on a large scale (Ren et al. 2009) and in investigations requiring rapid data analysis (Kemper and Sommer 2002, 2003; McCarthy and Humphries 2012). Thus, there is a need to develop a simple method for detecting trace metal contamination (considering as few environmental factors as possible) to guide more extensive and costly geochemical analyses.
Here, we hypothesized that dissolved—relative to sediment—concentrations of major elements associated with secondary oxides and oxyhydroxides in AMD-contaminated environments, could serve as good proxies for detecting AMD-related trace metal contamination in streams. To investigate our hypothesis, we calculated Fe and Al ‘dilution factors’ by dividing stream water concentrations (mg/L) of Fe and Al by their respective overbank sediment concentrations (mg/L), and then we mapped these [stream water/overbank sediment] dilution factors with dissolved trace metal concentrations in the study site. Fe and Al were selected for the calculation of dilution factors because they are the most common major elements associated with AMD (Alpers et al. 1994; Bigham and Nordstrom 2000; Nordstrom 2020) and were dominant in overbank sediments in the study site to a much larger extent than Mn.
The objectives of this study were to: characterize stream water chemistry at the study site, assess the extent of metal contamination in the study site based on enrichment factors (EFs) and contamination factors (CFs), and evaluate the potential of Fe and Al dilution factors for detecting trace metal contamination in the study site. The advantages of using Fe and Al to detect AMD-related trace metal contamination in streams are that they are major elements in AMD-contaminated environments and, thus, can be analyzed with greater precision than trace metals, which are typically less precise because they are closer to the detection limit of the analytical equipment (Hall 1998) and would require the analysis of dissolved and sediment concentrations of only two elements (i.e. Fe and Al), as opposed to a number of AMD-related trace metals, thus, potentially and markedly reducing the cost and time of laboratory analysis.
Materials and Methods
Study Area
Description of the Study Site
The Blesbokspruit River (Fig. 1) is situated in the Witbank Coalfield and forms part of the Olifants River catchment in South Africa. It is located ≈5 km northwest of the town of Emalahleni, Mpumalanga. The Blesbok Colliery, which has been mined by CCCR Commodities (2022) since 2020, is currently operating there. The main features of the study site include four upstream acid ponds and a wetland ≈3 km downstream of the acid ponds. The acid ponds were constructed to reduce the impact of polluted underground mine-water on the Blesbokspruit River (Bell et al. 2001) and are considered to be a source of AMD. The Blesbokspruit River was deemed suitable for study based on previous AMD studies at this site (Bell et al. 2001, 2002; Netshitungulwana et al. 2013). The climate of the area and its surroundings, based on the Köppen climate classification, is Cwb (temperate, dry winter, warm temperature). The average temperature during autumn (the season when we did our sampling) is 13–16 °C and average rainfall is 15–45 mm (Climate-data.org 2022). Stream flow, at the time of sampling, was laminar. The river showed no signs of aquatic life and, with the exception of the wetland and acid pond areas, was only sparsely vegetated. Thus, the influence of biota and organic matter on trace metal attenuation was not considered.
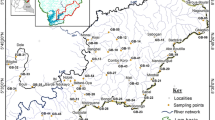
Localities for stream water sampling with- (red dots) and without (purple dots) overbank sediment samples along the Blesbokspruit River, Mpumalanga, South Africa. Stream water samples were collected at two sites approx. 5 m apart at each of the eleven different localities. Similarly, overbank sediment samples were collected at two sites roughly 5 m apart at each of the six different localities. Flow direction is indicated by the black, dashed arrow. Also shown is a wetland (green dash lines) and acid ponds (yellow rectangle)
Geology
The Witbank Coalfield is located within the northern portion of the Main Karoo Basin and by 2014, it was one of the major suppliers (yielding > 50%) of saleable coal in South Africa (Hancox and Götz 2014). The study area is characterized largely by outcrops of the Vryheid Formation, which forms part of the Permian Ecca Group of the Karoo Supergroup (SACS 1980), and several intrusive dolerite dykes and sills (Cairncross 2001). Rock sequences of the Vryheid Formation in the study area consists mainly of siltstone, sandstone, carbonaceous shale, five bituminous coal seams, and minor amounts of conglomerate (Cairncross 2001). The strata of the Witbank Coalfield are dominated by quartz and kaolinite, with trace to minor concentrations of pyrite, calcite, and dolomite (Azzie 2002; Pinetown 2003). Pyrite, which was present in percentages between 0.1 and 37.1%, was determined to be the dominant sulfide in these strata (Pinetown 2003). In terms of major element geochemistry, the Witbank Coalfield strata are dominated by SiO2 and Al2O3, with minor to trace amounts of Fe2O3, S, and CaO. With respect to trace element geochemistry, the strata are dominated by Cr with minor to high concentrations of Ni, Zn, Co, and Cu, and trace to minor concentrations of Pb (Cairncross 2001; Pinetown 2003).
Field Sampling and Analyses
Sampling was conducted during autumn (one of the driest seasons in the study area and its surroundings) and, thus, under base flow hydrological conditions. Base flow is defined as the portion of stream flow not directly generated by excess precipitation (Price 2011). The sampling was conducted in the dry season to minimize the influence of rainfall on the dilution factors. In addition to dilution, increased rainfall can also mobilize stored AMD (and related trace metals) in surface waters in the vicinity of coal mines (Jewiss et al. 2020) and thus undermine the utility of dilution factors when sampling is done during wet seasons. The irregular sampling interval was due mainly to water-logged sediments in the wetland between sample localities #3 and #6, sewerage contamination, and, at the time of sampling, bush burning between sample localities #6 and #7 (Fig. 1). Thus, water and sediment samples were sparse.
Data sparsity is occasionally unavoidable in geological studies (Davis 2002) and is a common scenario in environmental studies (e.g. n = 6 in Ayora et al. 2022; n = 5 in Fitzsimons and Courtney 2022; n = 10 in Petrini et al. 2016). However, Davis (2002) argued that sparse data can still be useful when handled with suitable statistical analyses and assessments of uncertainty. Therefore, the sparsity of data in our case was addressed by applying statistical analyses that are suitable for small datasets and the associated uncertainty was quantified using statistical significance (p) and confidence intervals (CIs).
Water Samples
Twenty-two stream water samples were collected (at a depth of ≈10 cm) at 11 different locations ≈0.2–1.5 km apart (i.e. two samples collected ≈5 m apart at each location). This was done because ion concentrations at adjacent locations along a stream can vary considerably during periods of low flow (i.e. during dry seasons; Floriancic et al. 2019). Bell et al. (2001) found that dissolved metal concentrations more than 6 km downstream of the acid ponds were almost negligible due to the alkalizing and neutralizing effect of the Prison Stream tributary, so only the first 6 km of the Blesbokspruit River downstream of the acid ponds were sampled. Sampling was conducted upstream from sample location #1 towards the acid pond contamination source (Fig. 1) to ensure minimal disturbance and cross-contamination downstream.
Physico-chemical parameters (pH and EC) were measured in situ using a HANNA 9828 multisensor probe and Mettler Toledo portable pH meter. Stream water samples were filtered using 0.45 µm nylon syringe filters, preserved using 10% HNO3 and kept in cool storage (U.S. EPA 1983) for analysis by ICP-AES/MS. ICP-AES/MS was used for elemental analysis because it provides low to very low (μg/L to ng/L) detection limits, which are crucial for analysis of trace metals in environmental studies. Elements were measured using prepared calibration solutions and quality control methods based on U.S. EPA guidelines (Stellenbosch University 2022). Al, Fe, Mn, Co, Ni, Cu, Zn, Pb, Cr, and Cd were selected for analysis because they are commonly associated with AMD (España 2007; Nieto et al. 2007; Sengupta 1993). Samples meant for sulfate analysis were filtered using 0.45 µm nylon syringe filters and kept cool (U.S. EPA 1983) prior to analysis by the Skalar Bluevision™ discrete analyzer.
Overbank Sediment Samples
Twelve overbank sediment samples were collected at six different locations (i.e. two samples collected ≈5 m apart at each location) along the Blesbokspruit River (Fig. 1). We collected samples at each location because Walling and He (1998) found considerable spatial variability of overbank sediment. Samples were collected at a depth of ≈10 cm, ≈1 m distance from the active stream. The advantages of using overbank (floodplain) sediments are that large quantities of sample are easy to collect and that in mine and industrially polluted areas, they may represent the most important secondary source of metal pollutants (Macklin and Klimek 1992). The overbank sediment samples were air-dried and stored in high density polyethylene plastic bags at room temperature. Samples meant for XRD Rietveld analysis were milled to size < 75 µm and analyzed using the Bruker D8 Advance Diffractometer (Brime 1985).
Samples meant for chemical analysis were sieved to < 63 µm size fraction because the trace metals of interest (Co, Ni, Cu, Zn, Pb, Cr, and Cd) are typically concentrated in this size fraction (Förstner and Salomons 1980). Sediment samples were treated with reverse aqua regia (3 HNO3: 1 HCl) and were digested using a Mars™ microwave digestion system to determine ‘near-total’ metal contents. ‘Near-total’ digestion (which does not include trace metals hosted in the crystalline lattice of primary minerals) was preferred because it represents the proportion of metals that is environmentally extractable (Shahbazi and Beheshti 2019). ICP-AES was used to analyze Fe and Al in sediments because it is suitable for major to minor elements while ICP-MS was used to analyze Mn, Sr, Ni, Cu, Zn, Pb, Cr, and Cd (which were present in much lower concentration ranges than Fe and Al) because it is more suitable for analysis of trace to ultra-trace elements (Stellenbosch University 2022).
Quality Control and Censored Values
Quality control and quality assurance (QC/QA) methods for stream water included the use of laboratory blanks and field- and analytical duplicates. The QC/QA methods for sediments included the use of field-, laboratory- and analytical duplicates as well as soil certified reference material (CRM). Elements with precision of 20% or better were included in further data analysis (Ramsey 1998). Censored data were substituted with 1/2 the detection limit as recommended by the U.S. EPA (1998, 2000). The detection limits of the analysis per metal via ICP-AES were (in mg/L): Al (13.50) and Fe (4.50); and via ICP-MS were (in μg/L): Al (0.83), Fe (0.90), Mn (0.04), Co (0.03), Ni (0.34), Cu (0.47), Zn (1.85), Pb (0.02), Cr (0.62), Cd (0.003) and Sr (0.07).
Data Analysis
Summary Statistics
The median and median absolute deviation (MAD) were used as measures of central tendency and variance, respectively. The advantage of using the median and MAD, as opposed to the mean and standard deviation, is that they do not assume a normal distribution and, thus, are robust against outliers (Reimann and Filzmoser 2000). Robust methods were necessary for this work because of the presence of outliers and the sparse number of stream water (n = 22) and sediment (n = 12) samples.
Contamination Assessment Indices
Metal contamination along the Blesbokspruit River was assessed using enrichment factors (EF) (Hakanson 1980), contamination factors (CF) (Hakanson 1980), and element (Fe and Al) ‘dilution factors’ (introduced as part of this study).
Enrichment Factor (EF): The EF technique assesses the degree of metal enrichment in soils, sediments, and surface waters relative to background metal concentrations and a conservative element. The goal is to distinguish between enrichment resulting from natural processes and anthropogenic impact (El-Kady et al. 2019). EF was defined by Hakanson (1980) as:
where Msample and Mbackground, respectively, are the concentrations of the metal of interest in the sample and background data; and Xsample and Xbackground, respectively, are the concentrations of a conservative element in the sample and background data. Contamination classes based on the EF were defined according to Birch (2003) as: no enrichment (< 1); minor enrichment (1–3); moderate enrichment (3–5); moderately severe enrichment (5–10); severe enrichment (10–25); very severe enrichment (25–50), and extremely severe enrichment (> 50).
Here, the MAD was used to represent background concentrations (Esmaeili et al. 2014; Rezapour et al. 2022). Use of the MAD results in site-specific enrichment factors and, thus, may address some of the concerns around the use of regional or global backgrounds in calculations of the EF (e.g. Reimann and de Caritat 2000, 2005). A conservative element is one that is generally stable in soils and sediments and is largely of lithogenic provenance (Dan et al. 2014). The EF can generally be calculated using Al, Fe, Mn, Ti, Sc, or Sr as a conservative element (Altıkulaç and Turhan 2023; Fan et al. 2019; Gaberšek et al. 2022; Kicińska and Wikar 2021; Kowalska et al. 2018; Kükrer et al. 2020). Unlike Al, Fe, and Mn which are commonly associated with coal mine drainage (Equeenuddin et al. 2013; Seo et al. 2017; Silva et al. 2011), aqueous Sr concentrations are generally associated with the natural weathering of geogenic materials along a river or streambed (Cánovas et al. 2007; Cao et al. 2020; Lenoble et al. 2013). Thus, Sr was considered a suitable conservative element.
Contamination factor (CF): The CF evaluates the anthropogenic component of trace metal concentrations in a study site based on the ratio of metal concentration in the sample to background concentrations of that metal. The CF was defined by Hakanson (1980) as:
where Csample and Cbackground represent the concentration of a dissolved metal in the sample and background concentrations (here, the MAD) of that metal, respectively. Contamination classes based on the CF were defined according to Hakanson (1980) as: no to low contamination (< 1.5); low contamination (1.5–2); moderate contamination (2–4); high contamination (4–8); very high contamination (8–16); extremely high contamination (16–32), and ultra-high contamination (> 32).
Element (Fe and Al) dilution factor: Dilution factors of Fe and Al are proposed in this present study and are defined here as:
-
Fe dilution factor = Festream water/Fesediment, and
-
Al dilution factor = Alstream water/Alsediment, respectively,
where Festream water and Alstream water represent Fe and Al concentrations in the stream water; and Fesediment and Alsediment represent Fe and Al concentrations in the overbank sediments. Contamination classes were defined according to the median ± MAD concentrations of the Fe and Al dilution factors and are represented as percentages (Supplemental Table S1).
Spatial distribution maps were generated using untransformed data of single element concentrations overlain with contamination classes based on the dilution factors of Fe and Al for comparison. The Fe and Al dilution factors and individual trace metal concentrations were not transformed because they do not originate from the same, closed composition and, thus, were considered independent (Reimann and de Caritat 2017; Reimann and Filzmoser 2000). Correlations among the calculated (stream water/overbank sediment) Fe and Al dilution factors and dissolved trace metal concentrations were evaluated using Spearman’s (1904) rank correlation analysis (Reimann and Filzmoser 2000). The CIs of correlations and hierarchical cluster analysis (HCA) based on the average linkage (between groups) method and squared Euclidean distance of trace metal concentrations were used to assess the robustness of the median ± MAD concentrations of the Fe and Al dilution factors for classifying AMD-related trace metal contamination in the streams. Centered log-ratio (clr) transformation was performed using CoDaPack (Comas-Cufí and Thió-Henestrosa 2011) and used to ‘open’ the ‘closed’ geochemical data (Aitchison 1981, 1986) prior to cluster analysis. Clr transformation was considered suitable because it produces results of better meaning (Carranza 2011) without requiring back-transformation to the original values for interpretation, as is necessary for isometric log-ratio (ilr) transformation (Egozcue and Pawlowsky-Glahn 2005).
Results and Discussion
Mineralogy
The results of XRD Rietveld analysis and ICP-AES/MS analysis of overbank sediments along the Blesbokspruit River are shown in Table 1. According to Table 1, the Blesbokspruit River overbank sediments were dominated by quartz (95.5–100%), with minor kaolinite proportions (0–4.5%), which is in agreement with Pinetown (2003). In addition, the overbank sediments showed elevated concentrations of amorphous Al (median = 4.18%) and Fe (median = 2.46%) oxides and oxyhydroxides, with minor concentrations of amorphous Mn (median = 201 mg/L) oxides and oxyhydroxides. Increased Fe (6.21%) and Mn (885 mg/L) content in sediments at sample location #1, compared to sample locations further upstream, is consistent with the precipitation of iron and manganese oxides and oxyhydroxides following the confluence of the Blesbokspruit River with the uncontaminated Prison Stream (Bell et al. 2001). The very high Al content (7.79%) in the acid pond (sample location #11) is consistent with periodic treatment of the ponds with soda ash (sodium carbonate) (Janse van Rensburg 2003), which can very efficiently precipitate Al from acidic waters (Masindi et al. 2017). Overall trends in metal content in sediments along the study site are consistent with the findings of Equeenuddin et al. (2013), Sahoo et al. (2017), and Santos et al. (2015), who noted much higher Fe and Al content in sediments in the vicinity of coal mines, compared to Mn contents.
Stream Water Chemistry and Metal Enrichment
According to Table 2, the Blesbokspruit River water had pH values ranging from acidic (< 3) to near-neutral (6.8) and EC values between 180 and 2240 (μS/cm). Dissolved sulfate ranged from 73.7–1530 (mg/L). Decreasing median major and trace metal concentrations in the Blesbokspruit River water were: Al (7.41 mg/L) > Mn (2.30 mg/L) > Fe (0.50 mg/L) > Sr (372 μg/L) > Zn (328 μg/L) > Ni (97 μg/L) > Co (71 μg/L) > Cu (2.64 μg/L) > Cr (0.86 μg/L) > Pb (0.71 μg/L) > Cd (0.58 μg/L). Median trace metal concentrations in the Blesbokspruit River waters appeared consistent with those documented by Miranda et al. (2022), who suggested that Zn and Ni typically have the greatest potential for deteriorating water quality because of their strong affinity for the aqueous phase. Among the elements typically considered conservative (Al, Fe, Mn, and Sr), Sr showed the lowest variability (MAD = 127 μg/L), supporting its use as a conservative element in the calculation of EFs (Loska et al. 2003). In addition, the higher Sr content in the acid pond (sample location #11; pH = 3.5), compared to sample locations #7, #8, #9, and # 10 (pH < 3.0), suggests that increased Sr content is less related to pH but is likely more associated with prolonged contact of stream water with streambed materials in the pond, as suggested by Cánovas et al. (2007).
Bell et al. (2001) reported median dissolved Al (113 mg/L), Fe (26.4 mg/L), Mn (12.4 mg/L), Cu (1.1 mg/L) Ni (1.9 mg/L), Pb (1.4 mg/L), and Zn (2.4 mg/L) concentrations for roughly the same portion of the Blesbokspruit River. When the water chemistry from the present study (Table 2) was compared with that of Bell et al. (2001), it is clear that dissolved metal concentrations in the Blesbokspruit River have markedly decreased over the last 20 years. A possible reason for this decrease may be the refurbishment of the Brugspruit Water Pollution Control Works treatment plant in 2014 (Rand Water 2014). The plant was originally built in 1997 by the South African Department of Water Affairs and Forestry to treat the acidic waters of the Brugspruit and Blesbokspruit Rivers. However, due to equipment theft, insufficient staff, and lack of maintenance, the plant had been non-operational for lengthy periods of time (Hobbs et al. 2008; McCarthy and Pretorius 2009).
According to the EFs in Table 3, waters along the Blesbokspruit River were characterized by: no to minor enrichment of Al, Mn, Co, Ni, Zn, and Cd; no to moderately severe enrichment of Pb, Cu, and Cr; and no to very severe enrichment of Fe. According to the CFs in Table 3, the study site was characterized by: no to moderate Mn, Co, Ni, Zn, and Cd contamination; no to high Al contamination; no to very high Cu, Pb, and Cr contamination; and no to ultra-high Fe contamination. Low pH, high EC, high sulfate, minor to moderately severe enrichment of trace metals, and ultra-high degrees of Fe contamination are clear indications that the Blesbokspruit River is still affected by AMD.
Spatial Variability of Metals
Concerning the spatial variability of dissolved trace metals (Supplemental Fig. S1), maximum trace metal concentrations (μg/L) occurred within 0.2–0.5 km downstream of the acid ponds for all of the studied metals except Cu (Supplemental Fig. S1f), which showed maximum dissolved trace metal concentrations ≈1.1 km downstream of the acid ponds (corresponding with the maximum kaolinite proportions in Table 1). The consistency between kaolinite and Cu was also documented by González Costa et al. (2017), who stated that higher clay contents in soils increase the bioavailability of Cu.
Trace metal (Co, Ni, Cu, Zn, Pb, Cr, and Cd) concentrations were notably lower at ≈2.8 km downstream of the acid ponds and lowest more than 4.6 km downstream of the acid ponds. Overall, dissolved trace metal concentrations typically decreased with increasing distance from the acid ponds, which is consistent with the findings of Bell et al. (2001). However, sample locality #6 showed dissolved trace metal concentrations lower than sample localities #5 and #4, which were located further downstream from the acid ponds. This is likely because samples at locality #6 were collected on the final day of sampling, following a day of rain that may have subsequently diluted the samples. The increased Mn, Co, and Ni content noted at sample location #1 is likely due to the dissolution of Mn, Co, and Ni that were scavenged by Fe oxide and oxyhydroxides in sediments (Table 1) at this location (Teixeira et al. 2001).
The spatial variability of metal concentrations in the waters of the Blesbokspruit River appeared to be strongly influenced by the pH, dissolved sulfate concentrations, the presence of a wetland, and the density and health of surrounding vegetation.
Role of pH: Trace metal concentrations (Supplemental Fig. S1d–j) exhibited a strongly inverse relationship with pH. This was expected because the hydroxyl groups on variable-charge oxide and clay mineral surfaces become deprotonated at near-neutral pH, thereby facilitating cation adsorption. At lower (acidic) pH levels, they become protonated, thereby facilitating cation desorption (Dzombak and Morel 1990; Sparks 2002). The relatively inverse relationships among dissolved Al, Fe, and Mn concentrations and pH (Supplemental Fig. S1a–c) suggest that these metal concentrations were strongly influenced by the solubility of hydroxides at low pH and, thus, they were likely in the form of Al3+, Fe3+, and Mn3+ (Cravotta 2006).
Role of dissolved sulfate: Trace metal concentrations (Supplemental Fig. S1d–j) in the acid ponds (sample locality #11 and in the study site, the supposed source of contamination) were notably lower than expected. This may be related to the much lower dissolved sulfate concentrations in the acid ponds compared to sample locality #10, 0.2 km downstream of the acid ponds (Table 2) since elevated concentrations of dissolved sulfate can increase dissolved metal concentrations via the formation of soluble metal sulfate complexes (Cravotta 2006). The higher pH (3.5) and lower dissolved sulfate content (216 mg/L) in the acid ponds compared to sample location #10 (pH = 2.6 and 1530 mg/L), is consistent with periodic treatment of the acid pond with soda ash (Janse van Rensburg 2003). Soda ash can cause the formation of insoluble sulfate complexes, which precipitate and settle out of solution (Masindi et al. 2017).
Role of the wetland and surrounding vegetation: The wetland was crucial in trace metal attenuation along the Blesbokspruit River, having recorded some of the lowest dissolved trace metal concentrations in the study site (Table 2). These findings were similar to those of Bell et al. (2001), who observed subdued trace metal concentrations in the Blesbokspruit wetland, relative to measurements further upstream. Metal attenuation in the wetland may be attributed to several processes, including settling and sedimentation of particulate‐bound metals, phytoextraction (uptake by and accumulation in plants), reduction and oxidation by bacteria, and adsorption to oxides, oxyhydroxides, clays, and organic matter present in the wetland (Sheoran and Sheoran 2006).
Wetland plants at sample locality #4 were noticeably drier than plants along other sections of the wetland. Water deficiency in plants can negatively affect their photosynthetic efficiency and root activity (Oguz et al. 2022; Peng et al. 2022), both of which play important roles in phytoextraction (Cassina et al. 2012; Wu et al. 2017). Thus, the dryness of wetland plants at sample locality #4 provides a possible explanation for the increase in trace metal concentrations at this location.
The increased biomass of plants is known to enhance the phytoextraction of trace metals and other pollutants (Sheoran et al. 2016). Thus, the dense vegetation around the acid ponds (sample locality #11) may provide an additional explanation for why the acid ponds themselves do not record the highest dissolved trace metal concentrations in the study site. Dense vegetation around the acid ponds may also explain why dissolved Pb (Supplemental Fig. S1h) (which unlike metals such as Co, Ni, Cu, Zn, Cd, and Cr, forms insoluble sulfate complexes and, thus, was expected to increase due to low sulfate concentrations in the acid ponds) showed a decrease similar to Co, Ni, Cu, Zn, Cd, and Cr in the acid ponds.
Element (Al and Fe) Dilution Factors
Contamination Assessment Indices
Using median ± MAD concentrations as the basis for classification (Supplemental Table S2), sample localities #1, #7, #8, #9, #10, and #11 (for which Al and Fe dilution factors were calculated) were grouped according to Al and Fe dilution factors, respectively, into levels of low (< 0.05%), moderate (0.05–0.13%), and high (> 0.13%) levels of trace metal contamination according to Al dilution factor, and into low (< 0.10%), moderate (0.10–0.13%), and high (> 0.13%) levels of trace metal contamination according to Fe dilution factor. Thus, according to the median ± MAD concentrations of the Al dilution factors, sample localities #1 and #11 were classified as ‘low’ trace metal contamination, sample localities #7, #8 and #9 as ‘moderate’ trace metal contamination and sample locality #10 as ‘high’ trace metal contamination (Fig. 2a). Similarly, according to median ± MAD concentrations of the Fe dilution factors, sample localities #1 and #11 were classified as ‘low’ trace metal contamination, sample localities #7, #8 and #10 as ‘moderate’ trace metal contamination, and sample locality #9 as ‘high’ trace metal contamination (Fig. 2b). Additional dilution factor maps are provided in Supplemental Fig. S2.
When the results of the classifications are compared with the HCA performed on dissolved trace metal concentrations (Fig. 3), samples classified by both the Fe and Al dilution factors as ‘low’ trace metal contamination correspond with cluster one, while samples classified as ‘moderate’ and ‘high’ trace metal contamination correspond with cluster two.
Correlation Analysis
Table 4 shows the Spearman’s rank correlations of aqueous trace metals with dilution factors, stream pH, and aqueous Fe and Al. The strengths of the correlations (r) were interpreted according to Dancey and Reidy (2017); thus, r = 1 indicates perfect correlation, r = 0.7–0.9 strong correlation, r = 0.4–0.6 moderate correlation, r = 0.1–0.3 weak correlation, and r = 0 no correlation. Strong positive correlations of the Al dilution factors with Co (r = 1.00, CI > 99%), Ni (r = 0.89, CI > 95%), Pb (r = 0.77, CI > 93%), Cr (r = 0.77, CI > 93%), Zn (r = 0.71, CI > 88%), and Cd (r = 0.71, CI > 88%) in Table 4, suggest the usefulness of Al dilution factors for detecting not only other lithophile metals (such as Cr), but also siderophile metals (such as Ni and Co) and chalcophile metals (such as Cd, Zn, and Pb). Similarly, the strong positive correlations of the Fe dilution factors with Cd (r = 1.00, CI > 99%), Ni (r = 0.94, CI > 99%), Co (r = 0.71, CI > 88%), and Zn (r = 0.71, CI > 88%) suggest the usefulness of Fe dilution factors for detecting not only siderophile metals (such as Ni and Co), but also chalcophile metals (such as Cd and Zn).
The relatively weak correlation between dissolved Cu and the Al dilution factors (r = 0.49) compared to the correlation between dissolved Cu and Fe dilution factors (r = 0.60) may be related to the influence of kaolinite (Table 1), which was fairly consistently associated with dissolved Cu at the study site (Supplemental Fig. S1f). González Costa et al. (2017) found that, compared to other clays, kaolinite has a high affinity for Cu. The increased affinity of kaolinite for Cu when coated with Fe-oxides (Osei and Singh 2000; Zhuang and Yu 2002) provides a possible explanation for the higher correlation observed between Cu and the Fe dilution factors (r = 0.60), compared to the Al dilution factors (r = 0.49).
Trace metal correlations with stream pH and aqueous Fe and Al are generally stronger than those with the dilution factors. However, compared to stream pH and aqueous Fe and Al, the dilution factors show stronger correlations with aqueous Co and Cd and comparable correlations with aqueous Ni. Strong correlations with Co, Cd, and Ni suggest that the dilution factors have a better potential for detecting mobile metals than relatively immobile metals such as Cu, Pb, and Cr (Covelo et al. 2008). Efficient detection and monitoring of Cd, in particular, is an important benefit of the ‘dilution factor’ method because of its mobility and hazardous effects on environmental and human health (Kicińska 2019, 2021).
Robustness of the Fe and Al Dilution Factors
Calculated Fe and Al dilution factors were moderate to strongly correlated with dissolved trace metals and showed CIs above 88% for all but Cu, which showed weaker correlation (Table 4). In addition, ‘low’, ‘moderate,’ and ‘high’ trace metal contamination groupings based on median ± MAD concentrations for both Fe and Al correspond well with sample groupings determined by the HCA based on clr-transformed dissolved trace metal concentrations (Fig. 3). This similarity in sample classifications suggests that the Fe and Al dilution factors and their median ± MAD statistics are fairly robust for the classification of AMD-related trace metal contamination in stream water affected by coal mining. However, the robustness of the dilution factors for detecting aqueous trace metal contents may be affected by increased rainfall during wetter seasons and by associated dilution and mobilization processes, as mentioned in the methodology. Thus, further investigation into the effects of seasonal change on the robustness of dilution factors for detecting aqueous trace metal contents is warranted.
Conclusions
The Blesbokspruit River was characterized by low pH, high EC, high sulfate, minor to moderately severe enrichment of trace metals, and ultra-high degrees of Fe contamination, all of which are strong indications that the river is affected by AMD. However, compared to previous studies, the quality of the Blesbokspruit River waters has significantly improved over the last 20 years, with metal concentrations showing a significant decrease, overall.
The spatial variability of trace metals was seemingly largely dependent on the pH of the river water, dissolved sulfate concentrations, and the health and density of the wetland and surrounding vegetation. Dissolved trace metal concentrations were generally inversely related to pH and positively related to dissolved sulfate concentrations. In addition, trace metal concentrations were seemingly elevated near sparse, dry vegetation and considerably more attenuated near dense, lush vegetation.
Al and Fe dilution factors showed moderate to strong positive correlations with dissolved Co, Ni, Zn, Pb, Cr, and Cd. The consistency between sample classifications based on the median ± MAD concentrations of the Fe and Al dilution factors and the HCA suggests that dilution factors are relatively robust and, thus, may serve as potential proxies for trace metal contamination in an AMD-affected stream during the dry season. Thus, it is recommended that future studies explore the use of Fe and Al dilution factors for detecting trace metal contamination in other AMD-affected streams with some additional considerations such as the influence of variable mineralogical contents in overbank sediments and climate and seasonality on the usefulness of Fe and Al dilution factors in the study area.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abrahams J-LR, Carranza EJM (2023) Trace metal content prediction along an AMD (acid mine drainage)-contaminated stream draining a coal mine using VNIR—SWIR spectroscopy. Environ Monit Assess 195(11):1261. https://doi.org/10.1007/s10661-023-11837-y
Adeniyi AG, Emenike EC, Iwuozor KO, Okoro HK, Ige OO (2022) Acid mine drainage: the footprint of the Nigeria mining industry. Chem Afr 5(6):1907–1920. https://doi.org/10.1007/s42250-022-00493-3
Aitchison J (1981) A new approach to null correlations of proportions. J Int Assoc Math Geol 13:175–189. https://doi.org/10.1007/BF01031393
Aitchison J (1986) The statistical analysis of compositional data. In: Monographs on statistics and applied probability. Chapman and Hall, London
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 2019:1–14. https://doi.org/10.1155/2019/6730305
Alomary AA, Belhadj S (2007) Determination of heavy metals (Cd, Cr, Cu, Fe, Ni, Pb, Zn) by ICP-OES and their speciation in Algerian Mediterranean Sea sediments after a five-stage sequential extraction procedure. Environ Monit Assess 135(1–3):265–280. https://doi.org/10.1007/s10661-007-9648-8
Alpers CN, Blowes DW, Nordstrom DK, Jambor JL (1994) Secondary minerals and acid mine–water chemistry. In: Blowes DW, Jambor JL (eds) The environmental geochemistry of sulfide mine-wastes. Mineral Assoc Canada Short Course Series, vol 22. pp 247–270
Altıkulaç A, Turhan Ş (2023) Assessment of the levels of potentially toxic elements contained in natural bentonites collected from quarries in Turkey. ACS Omega 8(23):20979–20986. https://doi.org/10.1021/acsomega.3c01773
Ayora C, Carrero S, Bellés J, Basallote MD, Cánovas CR, Macías F (2022) Partition of rare earth elements between sulfate salts formed by the evaporation of acid mine drainage. Mine Water Environ 41(1):42–57. https://doi.org/10.1007/s10230-021-00803-0
Azzie BA (2002) Coal mine waters in South Africa: their geochemistry, quality and classification. PhD thesis (unpubl), University of Cape Town, South Africa
Bakirdere S, Yaman M (2008) Determination of lead, cadmium and copper in roadside soil and plants in Elazig, Turkey. Environ Monit Assess 136(1–3):401–410. https://doi.org/10.1007/s10661-007-9695-1
Bell FG, Bullock SET, Halbich TFJ, Lindsay P (2001) Environmental impacts associated with an abandoned mine in the Witbank Coalfield, South Africa. Int J Coal Geol 45(2–3):195–216. https://doi.org/10.1016/S0166-5162(00)00033-1
Bell FG, Hälbich TFJ, Bullock SET (2002) The effects of acid mine drainage from an old mine in the Witbank Coalfield, South Africa. Q J Eng Geol Hydrogeol 35:265–278
Bigham JM, Nordstrom DK (2000) Iron and aluminum hydroxysulfates from acid sulfate waters. Sulfate minerals: Crystallography, geochemistry, and environmental significance. Rev Mineral Geochem 40(1):303–350. https://doi.org/10.2138/rmg.2000.40.7
Birch G (2003) A scheme for assessing human impacts on coastal aquatic environments using sediments. In: Woodcoffe CD, Furness RA (eds), Coastal GIS, Proceedings of a Workshop. Centre for Maritime Policy, Wollongong University, Wollongong, NSW
Brime C (1985) The accuracy of X-ray diffraction methods for determining mineral mixtures. Mineral Mag 49(353):531–538. https://doi.org/10.1180/minmag.1985.049.353.06
Cairncross B (2001) An overview of the Permian (Karoo) coal deposits of southern Africa. J Afr Earth Sci 33(3–4):529–562. https://doi.org/10.1016/s0899-5362(01)00088-4
Campaner VP, Luiz-silva W, Machado W (2014) Geochemistry of acid mine drainage from a coal mining area and processes controlling metal attenuation in stream waters, southern Brazil. An Acad Bras Cienc 86(2):539–554. https://doi.org/10.1590/0001-37652014113712
Cánovas CR, Basallote MD, Macías F, Freydier R, Parviainen A, Pérez-López R (2022) Thallium distribution in an estuary affected by acid mine drainage (AMD): The Ría de Huelva estuary (SW Spain). Environ Pollut 306:119448. https://doi.org/10.1016/j.envpol.2022.119448
Cánovas CR, Olías M, Nieto JM, Sarmiento AM, Cerón JC (2007) Hydrogeochemical characteristics of the Tinto and Odiel Rivers (SW Spain) factors controlling metal contents. Sci Total Environ 373(1):363–382. https://doi.org/10.1016/j.scitotenv.2006.11.022
Cao X, Wu P, Zhou S, Xie F, Rong R (2020) Trace elements geochemical characteristics of reservoir sediments affected by acid mine drainage. J Jilin Uni (Earth Sci Ed) 50(4):1112–1126
Carranza EJM (2011) Analysis and mapping of geochemical anomalies using logratio-transformed stream sediment data with censored values. J Geochem Explor 110(2):167–185. https://doi.org/10.1016/j.gexplo.2011.05.007
Cassina L, Tassi E, Pedron F, Petruzzelli G, Ambrosini P, Barbafieri M (2012) Using a plant hormone and a thioligand to improve phytoremediation of Hg-contaminated soil from a petrochemical plant. J Hazard Mater 231–232:36–42. https://doi.org/10.1016/j.jhazmat.2012.06.031
CCCR Commodities (2022) Blesbok colliery. https://cccr.co.za/blesbok-colliery. Accessed 26 Sept 2023
Climate-data.org (2022) Climate Emalahleni (South Africa). https://en.climate-data.org/africa/south-africa/mpumalanga/emalahleni-641/. Accessed 24 May 2022
Comas-Cufí M, Thió-Henestrosa S (2011) CoDaPack 2.0: a stand-alone, multi-platform compositional software. In: Egozcue JJ, Tolosana-Delgado R, Ortego MI (eds) CoDaWork’11: 4th International Workshop on Compositional Data Analysis. Sant Feliu de Guíxols
Covelo EF, Matías JM, Vega FA, Reigosa MJ, Andrade ML (2008) A tree regression analysis of factors determining the sorption and retention of heavy metals by soil. Geoderma 147(1–2):75–85. https://doi.org/10.1016/j.geoderma.2008.08.001
Cravotta CA (2006) Relations among pH, sulfate, and metals concentrations in Anthracite and Bituminous coal-mine discharges, Pennsylvania. In: Proceedings of the 23rd Ann Meet Am Soc Min Reclam 1 (June 2006), pp 378–404. https://doi.org/10.21000/jasmr06020378
Dan SF, Umoh UU, Osabor VN (2014) Seasonal variation of enrichment and contamination of heavy metals in the surface water of Qua Iboe River Estuary and adjoining creeks, South-South Nigeria. J Oceanogr Mar Sci 5(6):45–54. https://doi.org/10.5897/joms2013.0103
Dancey CP, Reidy J (2017) Statistics without maths for psychology. Pearson Education, London
Davis JC (2002) Statistics and data analysis in geology. John Wiley and Sons, New York
Dzombak DA, Morel FMM (1990) Surface complexation modeling. Hydrous Ferric Oxide. Wiley, New York
Egozcue JJ, Pawlowsky-Glahn V (2005) Groups of parts and their balances in compositional data analysis. Math Geol 37:795–828. https://doi.org/10.1007/s11004-005-7381-9
El-Kady AA, Wade TL, Sweet ST, Klein AG (2019) Spatial distribution and ecological risk assessment of trace metals in surface sediments of Lake Qaroun, Egypt. Environ Monit Assess 191(7):413. https://doi.org/10.1007/s10661-019-7548-3
Equeenuddin SM, Tripathy S, Sahoo PK, Panigrahi MK (2013) Metal behavior in sediment associated with acid mine drainage stream: role of pH. J Geochem Explor 124:230–237. https://doi.org/10.1016/j.gexplo.2012.10.010
Esmaeili A, Moore F, Keshavarzi B, Jaafarzadeh N, Kermani M (2014) A geochemical survey of heavy metals in agricultural and background soils of the Isfahan industrial zone, Iran. Catena 121:88–98. https://doi.org/10.1016/j.catena.2014.05.003
España JS (2007) The behavior of iron and aluminum in acid mine drainage: speciation, mineralogy, and environmental significance. In: Letcher TM (ed) Thermodynamics, solubility and environmental issues. Elsevier, Amsterdam
Fan SX, Wang XD, Lei J, Ran QQ, Ren YX, Zhou JH (2019) Spatial distribution and source identification of heavy metals in a typical Pb/Zn smelter in an arid area of northwest China. Hum Ecol Risk Assess 25:1661–1687
Fitzsimons O, Courtney R (2022) Characterisation of Pb/Zn tailings and drainage waters to inform post-closure water treatment strategies. Mine Water Environ 41(4):1118–1123. https://doi.org/10.1007/s10230-022-00898-z
Floriancic MG, Fischer BMC, Molnar P, Kirchner JW, Meerveld IHJ (2019) Spatial variability in specific discharge and streamwater chemistry during low flows: results from snapshot sampling campaigns in eleven Swiss catchments. Hydrol Process 33(22):2847–2866. https://doi.org/10.1002/hyp.13532
Förstner U, Salomons W (1980) Trace metal analysis on polluted sediments: assessment of sources and intensities. Environ Technol Lett 1(11):506–517
Gaberšek M, Watts MJ, Gosar M (2022) Attic dust: an archive of historical air contamination of the urban environment and potential hazard to health? J Hazard Mater 432:128745. https://doi.org/10.1016/j.jhazmat.2022.128745
Galán E, Gómez-Ariza JL, González I, Fernández-Caliani JC, Morales E, Giráldez I (2003) Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18(3):409–421. https://doi.org/10.1016/S0883-2927(02)00092-6
González Costa JJ, Reigosa MJ, Matías JM, Covelo EF (2017) Soil Cd, Cr, Cu, Ni, Pb and Zn sorption and retention models using SVM: variable selection and competitive model. Sci Total Environ 593–594:508–522. https://doi.org/10.1016/j.scitotenv.2017.03.195
Hakanson L (1980) An ecological risk index for aquatic pollution control a sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Hall GEM (1998) Analytical perspective on trace element species of interest in exploration. J Geochem Explor 61(1–3):1–19. https://doi.org/10.1016/S0375-6742(97)00046-0
Hancox PJ, Götz AE (2014) South Africa’s coalfields—a 2014 perspective. Int J Coal Geol 132:170–254. https://doi.org/10.1016/j.coal.2014.06.019
Hobbs P, Oelofse SHH, Rascher J (2008) Management of environmental impacts from coal mining in the Upper Olifants River Catchment as a function of age and scale. Int J Water Resour Dev 24(3):417–431. https://doi.org/10.1080/07900620802127366
Janse van Rensburg R (2003) A long-term acid mine drainage water management strategy for South Witbank Colliery, Mpumalanga. Unpublished MSc Thesis, University of Johannesburg
Jewiss C, Craw D, Pope J, Christenson H, Trumm D (2020) Dilution processes of rainfall-enhanced acid mine drainage discharges from historic underground coal mines, New Zealand. Mine Water Environ 39(1):27–41. https://doi.org/10.1007/s10230-019-00650-0
Kemper T, Sommer S (2002) Estimate of heavy metal contamination in soils after a mining accident using reflectance spectroscopy. Environ Sci Technol 36:2742–2747
Kemper T, Sommer S (2003) Mapping and monitoring of residual heavy metal contamination and acidification risk after the Aznalcóllar mining accident (Andalusia, Spain) using field and airborne hyperspectral data. In: Habermeyer M, Müller A, Holzwarth S (eds) Proceedings of the 3rd EARSEL Imaging Spectroscopy, CD‐ROM ISBN 2‐908885‐56‐5. pp 333–343
Kicińska A (2019) Environmental risk related to presence and mobility of As, Cd and Tl in soils in the vicinity of a metallurgical plant—long-term observations. Chemosphere 236:124308. https://doi.org/10.1016/j.chemosphere.2019.07.039
Kicińska A (2021) Physical and chemical characteristics of slag produced during Pb refining and the environmental risk associated with the storage of slag. Environ Geochem Health 43(7):2723–2741. https://doi.org/10.1007/s10653-020-00738-5
Kicińska A, Wikar J (2021) Ecological risk associated with agricultural production in soils contaminated by the activities of the metal ore mining and processing industry—example from southern Poland. Soil Tillage Res 205:104817. https://doi.org/10.1016/j.still.2020.104817
Kos S, Zupančič N, Gosar M, Miler M (2022) Solid carriers of potentially toxic elements and their fate in stream sediments in the area affected by iron ore mining and processing. Minerals 12(11):1424. https://doi.org/10.3390/min12111424
Kowalska JB, Mazurek R, Gąsiorek M, Zaleski T (2018) Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—a review. Environ Geochem Health 40(6):2395–2420. https://doi.org/10.1007/s10653-018-0106-z
Kükrer S, Erginal AE, Kılıç Ş, Bay Ö, Akarsu T, Öztura E (2020) Ecological risk assessment of surface sediments of Çardak Lagoon along a human disturbance gradient. Environ Monit Assess 192(6):359. https://doi.org/10.1007/s10661-020-08336-9
Kumar M, Srivastava MK, Kishor K, Singh AK (2023) An assessment of the environmental impact of coal mining through acid mine drainage and soil degradation from Makum Coalfields, Upper Assam, India: a case study. J Geol Soc India 99(8):1113–1120. https://doi.org/10.1007/s12594-023-2437-3
Lenoble V, Omanović D, Garnier C, Mounier S, Donlagić N, Le Poupon C, Pižeta I (2013) Distribution and chemical speciation of arsenic and heavy metals in highly contaminated waters used for health care purposes (Srebrenica, Bosnia and Herzegovina). Sci Total Environ 443:420–428. https://doi.org/10.1016/j.scitotenv.2012.10.002
Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A (2003) Assessment of arsenic enrichment of cultivated soils in southern Poland. Pol J Environ Stud 12(2):187–192
Macklin MG, Klimek K (1992) Dispersal, storage, and transformation of metal contaminated alluvium in the upper Vistula basin, southwest Poland. Appl Geogr 12:7–30
Manahan SE (1991) Environmental chemistry. Lewis Publishers, Chelsea, MI. Manceau
Masindi V, Akinwekomi V, Maree JP, Muedi KL (2017) Comparison of mine water neutralisation efficiencies of different alkaline generating agents. J Environ Chem Eng 5(4):3903–3913. https://doi.org/10.1016/j.jece.2017.07.062
McCarthy TS, Humphries MS (2012) Contamination of the water supply to the town of Carolina, Mpumalanga. S Afr J Sci 109(9/10):10. https://doi.org/10.1590/sajs.2013/20120112
McCarthy TS, Pretorius E (2009) Coal mining on the Highveld and its implications for future water quality in the Vaal River system. In: Proceedings of the International Mine Water Conf, Pretoria. pp 56–65
Miranda LS, Ayoko GA, Egodawatta P, Goonetilleke A (2022) Adsorption-desorption behavior of heavy metals in aquatic environments: influence of sediment, water and metal ionic properties. J Hazard Mater 421:126743. https://doi.org/10.1016/j.jhazmat.2021.126743
Nascimento SC, Cooke DR, Townsend AT, Davidson G, Parbhakar-Fox A, Cracknell MJ, Miller CB (2023) Long-term impact of historical mining on water quality at Mount Lyell, western Tasmania, Australia. Mine Water Environ 42(3):399–417. https://doi.org/10.1007/s10230-023-00943-5
Netshitungulwana R, Yibas B, Novhe O, Motlakeng T (2013) Stream sediment geochemistry of the areas impacted by mining around Emalahleni (Witbank), South Africa: fingerprinting AMD potential point sources. IMWA 2013—Reliable Mine Water Technology for Sustainable Global Mining. Golden, CO, pp 17–22
Nieto JM, Sarmiento AM, Olías M, Canovas CR, Riba I, Kalman J, Delvalls TA (2007) Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ Int 33(4):445–455. https://doi.org/10.1016/j.envint.2006.11.010
Nordstrom DK (2020) Geochemical modeling of iron and aluminum precipitation during mixing and neutralization of acid mine drainage. Minerals 10(6):1–12. https://doi.org/10.3390/min10060547
Oguz MC, Aycan M, Oguz E, Poyraz I, Yildiz M (2022) Drought stress tolerance in plants: interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2:180–197. https://doi.org/10.3390/physiologia2040015
Ojonimi TI, Okeme IC, Phiri-Chanda T, Ameh EG (2021) Acid mine drainage (AMD) contamination in coal mines and the need for extensive prediction and remediation: a review. J Degrade Min Land Manage 9(1):3129–3136. https://doi.org/10.15243/jdmlm.2021.091.3129
Osei SA, Singh B (2000) Effect of selective removal of organic matter and iron oxides on the specific areas of some tropical soil clays. Ghana J Agric Sci 33:55–61. https://doi.org/10.4314/gjas.v33i1.1884
Pandit CM, Filippelli GM, Li L (2010) Estimation of heavy-metal contamination in soil using reflectance spectroscopy and partial least-squares regression. Int J Remote Sens 31(15):4111–4123. https://doi.org/10.1080/01431160903229200
Parker SR, Gammons CH, Jones CA, Nimick DA (2007) Role of hydrous iron oxide formation in attenuation and diel cycling of dissolved trace metals in a stream affected by acid rock drainage. Water Air Soil Pollut 181(2007):247–263. https://doi.org/10.1007/s11270-006-9297-5
Peng X, Li J, Sun L, Gao Y, Cao M, Luo J (2022) Impacts of water deficit and post-drought irrigation on transpiration rate, root activity, and biomass yield of Festuca arundinacea during phytoextraction. Chemosphere 294:133842. https://doi.org/10.1016/j.chemosphere.2022.133842
Petrini R, Cidu R, Slejko FF (2016) Thallium contamination in the Raibl mine site stream drainage system (Easters Alps, Italy). Mine Water Environ 35(1):55–63. https://doi.org/10.1007/s10230-015-0346-4
Pinetown KL (2003) Quantitative evaluation of minerals in coal deposits in the Witbank and Highveld Coalfields and potential impact on acid mine drainage. MSc thesis, University of the Free State, South Africa
Price K (2011) Effects of watershed topography, soils, land use, and climate on baseflow hydrology in humid regions: a review. Prog Phys Geogr 35(4):465–492. https://doi.org/10.1177/0309133311402714
Ramsey MH (1998) Sampling as a source of measurement uncertainty: techniques for quantification and comparison with analytical sources. J Anal Atomic Spectrom 1998(13):97–104. https://doi.org/10.1039/A706815H
Rand Water (2014) Rand Water Integrated Annual Report 2013–2014. p 230
Reimann C, de Caritat P (2000) Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ Sci Technol 34:5084–5091. https://doi.org/10.1021/es001339o
Reimann C, de Caritat P (2005) Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci Total Environ 337(2005):91–107
Reimann C, de Caritat P (2017) Establishing geochemical background variation and threshold values for 59 elements in Australian surface soil. Sci Total Environ 578:633–648. https://doi.org/10.1016/j.scitotenv.2016.11.010
Reimann C, Filzmoser P (2000) Normal and lognormal data distribution in geochemistry: death of a myth. Consequences for the statistical treatment of geochemical and environmental data. Environ Geol 39(9):1001–1014. https://doi.org/10.1007/s002549900081
Ren H, Zhuang DF, Sing AN (2009) Estimation of As and Cu contamination in agricultural soils around a mining area by reflectance spectroscopy: a case study. Pedosphere 19(6):719–726
Rezapour S, Asadzadeh F, Nouri A, Khodaverdiloo H, Heidari M (2022) Distribution, source apportionment, and risk analysis of heavy metals in river sediments of the Urmia Lake basin. Sci Rep 12(1):1–18. https://doi.org/10.1038/s41598-022-21752-w
SACS (South African Committee for Stratigraphy) (1980) Stratigraphy of South Africa. Part 1 (Compiler L.E. Kent). Lithostratigraphy of the Republic of South Africa, South West Africa/Namibia, and the Republics of Bophutatswana, Transkei and Venda. Handbook Geological Survey of South Africa
Sahoo PK, Tripathy S, Panigrahi MK, Equeenuddin SM (2017) Anthropogenic contamination and risk assessment of heavy metals in stream sediments influenced by acid mine drainage from a northeast coalfield, India. Bull Eng Geol Environ 76(2):537–552. https://doi.org/10.1007/s10064-016-0975-2
Santos MJ, Tarley CRT, Cunha I, Zapelini I, Galunin E, Bleinroth D, Vieira I, Abrão T (2015) Leachability of major and minor elements from soils and sediments of an abandoned coal mining area in Southern Brazil. Environ Monit Assess 187(3):1–13. https://doi.org/10.1007/s10661-015-4271-6
Schaider LA, Senn DB, Estes ER, Brabander DJ, Shine JP (2014) Sources and fates of heavy metals in a mining-impacted stream: temporal variability and the role of iron oxides. Sci Total Environ 490:456–466. https://doi.org/10.1016/j.scitotenv.2014.04.126
Schulz-Zunkel C, Krueger F (2009) Trace metal dynamics in floodplain soils of the River Elbe: a review. J Environ Qual 38(4):1349–1362. https://doi.org/10.2134/jeq2008.0299
Sengupta M (1993) Environmental impacts of mining: monitoring, restoration, and control. Lewis Publishers, Boca Raton, FL
Seo EY, Cheong YW, Yim GJ, Min KW, Geroni JN (2017) Recovery of Fe, Al and Mn in acid coal mine drainage by sequential selective precipitation with control of pH. Catena 148:11–16. https://doi.org/10.1016/j.catena.2016.07.022
Shahbazi K, Beheshti M (2019) Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Appl Sci 1(12):1–19. https://doi.org/10.1007/s42452-019-1578-x
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19(2):105–116. https://doi.org/10.1016/j.mineng.2005.08.006
Sheoran V, Sheoran AS, Poonia P (2016) Factors affecting phytoextraction: a review. Pedosphere 26(2):148–166. https://doi.org/10.1016/S1002-0160(15)60032-7
Silva LFO, Wollenschlager M, Oliveira MLS (2011) A preliminary study of coal mining drainage and environmental health in the Santa Catarina region, Brazil. Environ Geochem Health 33(1):55–65. https://doi.org/10.1007/s10653-010-9322-x
Skousen J (2014) Overview of acid mine drainage treatment with chemicals. In: Jacobs JA, Lehr JH, Testa SM (eds) Acid mine drainage, rock drainage, and acid sulfate soils: causes, assessment, prediction, prevention, and remediation. Wiley Online Library, New York
Skousen J, Rose A, Geidel G, Foreman J, Evans R, Hellier W (1999) Handbook of technologies for avoidance and remediation of acid mine drainage. Acid Drainage Technology Initiative (ADTI) of the U.S. Office of Surface Mining. National Mine Land Reclamation Center, WVU, Morgantown
Skousen JG, Ziemkiewicz PF, McDonald LM (2019) Acid mine drainage formation, control and treatment: approaches and strategies. Extr Ind Soc 6(1):241–249. https://doi.org/10.1016/j.exis.2018.09.008
Sparks DL (2002) Environmental soil chemistry. Academic Press, San Diego
Spearman C (1904) The proof and measurement of association between two things. Am J Psychol 100(3/4):441–471. https://doi.org/10.2307/1422689
Stellenbosch University (2022) ICP-MS-XRF. https://www.sun.ac.za/english/research-innovation/caf/units-laboratories/icp-ms-xrf. Accessed Oct 2023
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Mol Clin Environ Toxicol 101:133–164
Teixeira EC, Ortiz LS, Alves MFCC, Sanchez JCD (2001) Distribution of selected heavy metals in fluvial sediments of the coal mining region of Baixo Jacuí, RS, Brazil. Environ Geol 41(1–2):145–154. https://doi.org/10.1007/s002540100257
U.S. EPA (1983) Sample preservation. In: Methods for chemical analysis of water and wastes. EPA-600/4–79–020. Cincinnati, OH
U.S. EPA (1998) Guidance for data quality assessment: practical methods for data analysis. EPA/600/R-96/084, Office of Research and Development, Washington, DC
U.S. EPA (2000) Guidance for data quality assessment. Practical methods for data analysis. Office of Environmental Information, Washington, DC, p 219
Uysal K, Köse E, Bülbül M, Dönmez M, Erdoǧan Y, Koyun M, Ömeroǧlu Ç, Özmal F (2009) The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ Monit Assess 157(1–4):355–362. https://doi.org/10.1007/s10661-008-0540-y
Walling DE, He Q (1998) The spatial variability of overbank sedimentation on river floodplains. Geomorphology 24(2–3):209–223
Webster JG, Swedlund PJ, Webster KS (1998) Trace metal adsorption onto an acid mine drainage iron(III) oxy hydroxy sulfate. Environ Sci Technol 32(10):1361–1368. https://doi.org/10.1021/es9704390
Wilkin RT (2008) Contaminant attenuation processes at mine sites. Mine Water Environ 27(4):251–258. https://doi.org/10.1007/s10230-008-0049-1
Wu H, Wang X, He X, Zhang S, Liang R, Shen J (2017) Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci Total Environ 598:697–703
Zhao H, Xia B, Qin J, Zhang J (2012) Hydrogeochemical and mineralogical characteristics related to heavy metal attenuation in a stream polluted by acid mine drainage: a case study in Dabaoshan Mine, China. J Environ Sci (China) 24(6):979–989. https://doi.org/10.1016/S1001-0742(11)60868-1
Zhuang J, Yu GR (2002) Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals. Chemosphere 49(6):619–628. https://doi.org/10.1016/S0045-6535(02)00332-6
Acknowledgements
Funding for this research was provided by the South African National Space Agency (SANSA). SANSA has no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Funding
Open access funding provided by University of the Free State.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abrahams, JL.R., Carranza, E.J.M. Geochemical Characterization of an AMD-Affected Stream: Detection of Associated Trace Metal Contamination Using Element ‘Dilution Factors’. Mine Water Environ (2024). https://doi.org/10.1007/s10230-024-00982-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10230-024-00982-6