Abstract
The distribution of trace metals in active stream sediments from the mineralized Lom Basin has been evaluated. Fifty-five bottom sediments were collected and the mineralogical composition of six pulverized samples determined by XRD. The fine fraction (< 150 µm) was subjected to total digestion (HClO4 + HF + HCl) and analyzed for trace metals using a combination of ICP-MS and AAS analytical methods. Results show that the mineralogy of stream sediments is dominated by quartz (39–86%), phyllosilicates (0–45%) and feldspars (0–27%). Mean concentrations of the analyzed metals are low (e.g. As = 99.40 µg/kg, Zn = 573.24 µg/kg, V = 963.14 µg/kg and Cr = 763.93 µg/kg). Iron and Mn have significant average concentrations of 28.325 and 442 mg/kg, respectively. Background and threshold values of the trace metals were computed statistically to determine geochemical anomalies of geologic or anthropogenic origin, particularly mining activity. Factor analysis, applied on normalized data, identified three associations: Ni–Cr–V–Co–As–Se–pH, Cu–Zn–Hg–Pb–Cd–Sc and Fe–Mn. The first association is controlled by source geology and the neutral pH, the second by sulphide mineralization and the last by chemical weathering of ferromagnesian minerals. Spatial analysis reveals similar distribution trends for Co–Cr–V–Ni and Cu–Zn–Pb–Sc reflecting the lithology and sulphide mineralization in the basin. Relatively high levels of As were concordant with reported gold occurrences in the area while Fe and Mn distribution are consistent with their source from the Fe-bearing metamorphic rocks. These findings provide baseline geochemical values for common and parallel geological domains in the eastern region of Cameroon. Although this study shows that the stream sediments are not polluted, the evaluation of metal composition in environmental samples from abandoned and active mine sites for comparison and environmental health risk assessment is highly recommended.
Similar content being viewed by others
Introduction
Geochemical mapping surveys have been conducted in different parts of the world at various scales [1,2,3,4,5,6,7,8,9]. Although such mapping programmes were developed primarily for geochemical prospecting [10, 11], the same principles and techniques have been expanded to encompass environmental-related issues such as land use planning, agricultural development, environmental monitoring and medical geology [12,13,14,15,16,17]. The geochemical maps resulting from such surveys show the distribution (background) of the elements analyzed. The term ‘geochemical background’ was first used in exploration geochemistry [18] and a precise definition is yet to be universally accepted [19,20,21]. Commonly, background is used interchangeably with baseline or threshold value and may refer to element concentrations in real sample collectives due to natural processes in pristine areas or describe anthropogenic conditions [22, 23]. Considering its spatial and temporal variability, geochemical background represents the natural concentration range of an element in a given environmental medium [24]. Geochemical surveys often target diverse sampling media including rock, soil, sediment, surface water, groundwater, rain, plant and animals, with the aim of providing basic information for policymakers and industry purposes.
Stream sediments have been extensively used as a reliable medium in geochemical mapping investigations because they provide the composite sample of the catchment area upstream of the sampling point [8, 25,26,27,28,29,30]. This mixture of sediments, rock fragments and soils act not only as an ultimate sink for trace elements derived from within the catchment but are considered as sources of metals based on changes in environmental conditions which could pose pollution problems [31, 32]. Consequently, their geochemical composition is considered to be a representative of the drainage basin geology and an effective proxy for soil and groundwater [14, 33]. Nevertheless, the spatial distribution pattern of elemental levels in sediments is characterized by a high degree of diversity. Such spatial heterogeneity is due to myriad factors including the lithology and size of the basin, weathering processes, hydrological features and land use [34]. The natural weathering of mineral deposits, as well as human activities such as small-scale mining, can result in high concentrations of trace metals in stream sediments [35,36,37].
Most stream sediments surveys in Cameroon have focused on mineralization and provenance (e.g. [38,39,40,41,42,43]). Besides, a national geochemical mapping is yet to be implemented in Cameroon like in many countries since it is logistically demanding and considerably expensive. However, a regional geochemical survey can effectively reveal the geochemical characteristics of the sampled medium. In addition, baseline geochemical mapping of a watershed such as the Lom Basin which includes an important mining site is crucial for future environmental assessment. This region is an important prospective area for gold with extensive research having been carried out on the secondary alluvial gold and primary gold mineralization [44,45,46,47,48], soil quality [49, 50] and water quality [51]. On the other hand, there have been no studies on the geochemistry of active bottom sediments for environmental purposes. Thus, the determination of geochemical baseline is fundamental to setting guidelines for environmental management. In fact, geochemical mapping incorporating stream water and stream sediment is a holistic approach to understanding the bulk chemistry and the geochemical processes occurring within this heavily mineralized basin. Accordingly, Mimba et al. [52, 53] investigated the major ion and trace metal geochemistry of stream water in the Lom Basin and reported that the streams were not contaminated in spite of the past and ongoing mining activities.
The present study, therefore, focuses on the mineralogical and geochemical features of stream sediments from the lower Lom Basin. The purpose of this study is: (a) to characterize the mineralogical and trace metal composition of streambed sediments, (b) to evaluate the level of trace element contamination in sediments in comparison to local soils and Sub-Saharan Africa soil composition (c) to identify the sources of trace elements based on spatial distribution.
Study area
Regional setting, hydrology and climate
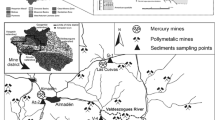
A 1:50,000 scale map showing the location and sampling sites in the Lom Basin is presented in Fig. 1. The study area is found in the Lom-and-Djerem Division, East Region of Cameroon which forms part of the Equatorial Rainforest Belt. It includes two major gold districts, Betare-Oya and Garoua Boulai, and covers a surface area of 2574.67 km2. Monotonous, gently undulating hills of altitude ranging from 600 to 1000 m above sea level extend throughout the area. This characteristic landform has led to the development of a dendritic drainage pattern (Fig. 1). The basin is drained by the Lom River and its tributaries. Some of the lower order streams have no water flow during the dry season. During the wet period, the stream discharge increases transporting most of the stream sediments within the catchment. The study area is covered mostly by shrubs and herbaceous savanna in the north and an evergreen forest in the south. High temperatures (average, 24.7 °C), rainfall (mean annual range, 1500–2000 mm) and humidity support the luxuriant vegetation cover and enhance deep weathering of rocks and the formation of iron-rich, red ferallitic soils. There is a lack of traditional seasons but instead a long dry season from December to May, a light wet season from May to June, a short dry season from July to August and a heavy wet season from September to November.
Geological setting and anthropogenic activities
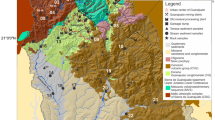
The Lom Basin constitutes part of the Precambrian basement of Cameroon which is divided into two significant lithostructural units; the Congo craton (CC) and the Central African Fold Belt (CAFB) [54]. The CAFB (~ 600 + 700 Ma) also known as the Pan-African belt of central Africa lies between the CC to the south and the Western Nigerian Shield to the north. This domain underlies Chad, Cameroon, Central African Republic and continues to parts of East Africa (Uganda and Sudan) [55, 56]. According to Castaing et al. [57], the evolution of this belt is due to the convergence and collision between the São Francisco–Congo cratons and the West African Craton, and a Pan-African mobile belt. In Cameroon, the present structure of the Pan-African belt can be attributed to the collision between the West Africa and Congo cratons [55].
Structurally, the CAFB is dominated by the Yaoundé Domain, Adamawa-Yadé Domain (AYD), and the Northwestern Cameroon Domain [55, 56, 58]. The AYD is the largest lithostructural unit of the CAFB in Cameroon. It is confined to the north by Tcholliré Banyo faults and to the south by the Yaoundé Domain (Fig. 2). This domain is marked by the development of high angle, strike-slip structures [59]. In addition, metavolcanic rocks of the Lom Basin have been identified as a major lithological group in the AYD (Fig. 2). The syn-to late-collisional calc-alkaline granitoids are ubiquitous within this domain. These rocks intrude orthogneisses representing the Paleoproterozoic basement which underwent extension and was probably dismembered during the Pan-African event. The Pan-African tectonism resulted in the formation of extensional basins including the Lom Basin [60, 61] underlain by three main units; volcaniclastic schists, metasedimentary rocks and the S-type granites [60, 62, 63]. The schists units are intercalated with quartzites and metaconglomerates and intruded by granitic plutons. The reworking of the Precambrian basement accounts for mineralization (especially gold) in the study area [55]. Consequently, alluvial gold is mined from the Lom River and its tributaries. During the last four decades, there has been a mining boom (exploration and exploitation) in the East Region of Cameroon, particularly in the districts of Betare-Oya, Batouri, Garoua-Boulai, Colomines and Kette [64, 65]. In this region, gold mining is artisanal and semi-mechanized. Gold is recovered from open pits, streambed sediments and weathered primary deposits (quartz veins) using primitive methods. Semi-mechanized exploitation often involves the use of heavy machinery. Stream sediments of the upper Lom Basin have Au concentrations of up to 450 ppm [66]. Besides small-scale mining, subsistence farming is a major form of livelihood amidst logging and timber.
Geology of Cameroon. a Geological map of Cameroon (modified from Toteu et al. [95] Faults: Tcholliré-Banyo Fault (TBF), Adamawa Fault (AF), Sanaga Fault (SF), and Kribi-Campo Fault (KCF) Cratons and mobile belts: West African Craton (WAC), Tanzanian Craton (TC), Kalahari Craton (KC), Congo Craton (CC), Adamaoua Shear Zone (ASZ). b Regional geological map of eastern Cameroon showing reported gold indications. c Geology of the lower Lom Basin
Materials and methods
Sampling and sample preparation
Stream sediments were sampled from lower order streams draining the southeastern part of the Lom Basin, at a density of one sample every 5–10 km (Fig. 1). Sampling was done preferably near stream confluences in order to cover the whole drainage network within the area. During sampling, care was taken to avoid areas of anthropogenic influences. Nonetheless, field observations such as potential sources of contamination, land use, and upstream geology were recorded. A total of 55 active stream sediment samples were collected based on the procedures from Salminen et al. [67]. About 3 kg (to ensure that sufficient fine-grained material would be available for analysis) of the upper layer (0–10 cm) of the stream sediment was collected using a hand trowel. The wet sediment was passed through first, a 300-µm, then 150-µm stainless steel sieves set to obtain the < 150-µm fraction. This retained portion was left to settle out and excess water carefully decanted. The drained < 150-µm fraction of stream sediment was placed in clean pre-labeled polyethylene bags and air dried. Duplicate samples were collected for each site.
Chemical analyses
In the laboratory, stream sediment samples were rinsed with Milli Q by shaking in an ultrasonic bath for 30 min each. They were then air dried and homogenized. Wet digestion and dissolution protocol (modified from Makishima and Nakamura [68]) for trace metals in the stream sediment samples was as follows. 0.15 mL of concentrated perchloric acid (60 wt% HClO4) and 0.3 mL of concentrated hydrofluoric acid (60 wt% HF) were added to 0.02 g of the sediment powder in a Teflon plastic bottle. The bottles were tightly capped and agitated in an ultrasonic cleanser for several hours to enhance the dissolution of samples. After complete decomposition, the bottles were uncorked, loaded on a ceramic hot plate and the samples were step-wise dried at 120, 170 and 200 °C, for 6 h at each step. Heating was carried out in a closed system. 0.2 mL of concentrated hydrochloric acid (35–37 wt% HCl) was then added and the bottle was agitated for 2 h to dissolve the degraded sample completely. Finally, the samples were dried at a temperature of 120 °C for 6 h to prevent the formation of iron oxides or hydroxides upon the final addition of nitric acid (HNO3). The samples were dissolved in 25 mL of 0.5 M HNO3 and stored in 50 mL polyethylene bottles for trace metal analysis.
Concentrations of Sc, V, Cr, Co, Ni, Cu, Zn, As, Se, Cd, Hg, and Pb in the sediments were determined using inductively coupled plasma mass spectrometry (ICP-MS) (ThermoScientific), Fe and Mn by atomic absorption spectroscopy (AAS) (contrrAA700) at the Laboratory of Volcanology and Geochemistry in Tokai University, Japan. The geochemical reference samples JA-3, JB-3 and JG-3 (Geological Survey of Japan) were used as standards. Internal standards and blanks were run at regular intervals in the analysis for quality control.
The pH of the stream sediments was measured following the procedure by the International Soil Reference and Information Center (ISRIC) [69]. A 1:2.5 ratio of solid to liquid was used. About 2 g of sediment powder was mixed with 5 mL of Milli Q in Teflon plastic bottles. The bottles were capped and agitated in an ultrasonic bath for 2 h. Prior to pH measurement, the mixture was shaken by hand and the pH of the supernatant suspension was read using a pH meter (LAQUAtwin), previously calibrated with buffer solutions of pH 4 and pH 7.
X-ray diffraction (XRD) analysis
The relative abundances of the main silicate and oxide minerals were determined semi-quantitatively on the bulk powder using a D8 ADVANCE TKK Diffractometer with automated divergence slit and monochromatic Cu-Kα radiation (4 kV–20 mV) at the Laboratory of Inorganic Chemistry and Material Science in Tokai University, Japan. Powders from 6 pulverised (< 150 µm) representative bottom sediment sub-samples were mounted with a random orientation on an aluminum sample holder. The powder was smoothened using a slide to obtain a uniform level suitable to the X-ray beam. Each sample was scanned from 10° to 80° 2θ with a 0.5 s step. The software BRUKER-binary V4 (.RAW) was used to provide a semi-quantitative estimation of mineral content based on the diffraction patterns.
Data processing
Statistical analyses
Statistical analyses were performed using Microsoft Excel and SPSS 20.0 for Windows. The statistical parameters minimum, median, mean and maximum measured the central tendency; while median absolute deviation (MAD), standard deviation, variance and coefficient of variance examined the statistical dispersion. Also, the dataset was tested for asymmetry using skewness. Univariate summary statistics showed that all measured elements were positively skewed. The geochemical data were then log-transformed to obtain a log-normal distribution. In addition, the data set was checked for outliers using Tukey boxplots [70] and the resultant data subset was used in threshold calculation as follows:
Multivariate statistical analyses (correlation matrix and factor analysis) were then applied to explore and investigate the data structure, decipher trends and relationship between variables; and infer the underlying factors influencing the stream sediment geochemistry.
Map production
Coloured geochemical maps of the data subset were drawn using the ESRI ArcMap 10.2 software package. For interpolation in a grid format, the inverse distance weight (IDW) technique was employed. A maximum of 15 neighboring samples was used for the estimation of each grid point and a power of 2 was chosen to achieve some degree of smoothing. The geochemical data were then classified based on the percentiles 5, 25, 50, 75, 90 and 98% and colour-coded according to this range. Highest concentrations were shown in hot colours while the lowest ranges were shown in cold colours. Also, graduated symbol plots of factor scores of the element associations obtained by factor analysis were produced to examine their relationship with the basin geology.
Results and discussion
Mineralogical composition
XRD analysis identified the following mineral phases: ubiquitous quartz (39–86%), moderate amounts of phyllosilicates (micas + clay minerals) and feldspars (Table 1). Rutile and gismondine were identified in only two samples (Table 1). This variability in mineralogy reflects the composition of the complex basement geology dominated by migmatitic gneisses, granites, metasedimentary and metavolcanic rocks (Fig. 2). The predominance of quartz in the sediments is likely due to reworking of sediments along flow paths. In a typical tropical basin like the study area, the high intensity of chemical weathering may also contribute to the modification of the sediment composition [71]. Additionally, hydraulic energy and sorting are also known to influence the mineral composition of sediments [72]. The samples GB15, GB20 and GB44 were collected in streams where the water flux was low. Thus, this accounts for the moderate phyllosilicates content in the bottom sediments.
Trace metal content and sediment quality assessment
Descriptive statistics of trace metal concentrations in stream sediments are presented in Table 2. Pronounced deviations between means, medians, standard deviations and MADs were observed. Also, all the selected metals were positively skewed implying the influence of extreme values, the presence of multiple populations and the effects of analytical precision or limits of detection of the data set [73]. The mean value of pH = 6.4 indicates near neutral conditions of the catchment. Elemental composition showed a wide variation which is likely generated by the physical and chemical weathering processes operating within the drainage basin [74, 75]. From the analytical results, Cd (2.65 µg/kg) had the lowest mean concentration followed by Hg (5.40 µg/kg), Se (48.55 µg/kg), Co (92.85 µg/kg) and As (99.40 µg/kg) (Table 2). These elements are usually present in trace amounts in rocks as reported in the mean background contents in the continental crust [76]. Iron and Mn had the highest mean concentrations of 28.325 and 442 mg/kg, respectively. When compared to regional stream sediment surveys in other Sub-Saharan Africa regions [26, 77], the Lom Basin sediments were depleted in all examined trace metals. However, average concentrations of the non-essential trace metals As (99.40 µg/kg), Hg (5.40 µg/kg), V (963.14 µg/kg) and Pb (151.59 µg/kg) were significantly higher than the levels (As = 22.3 µg/kg, Hg ≤ 0.01 µg/kg, V = 158.5 µg/kg and Pb = 12.3 µg/kg) reported by Taiwo and Awomeso [37] for sediment from the gold city of Ijeshaland. Also, all the trace metals analyzed showed a similar fingerprint in the stream waters of the study area characterized by low levels (< 1 µg/L) of V, Cr, Co, Cu, Zn, Cd, Pb and significant concentrations of Fe (20–5011 µg/L) and Mn (0.2–248 µg/L) [53]. Possible reasons for the depletion of these trace metals may be a reflection of the impoverished bedrock and the neutral pH which does not favour the dissolution and mobilization of the trace metals from the sulphide gold-quartz veins [78].
Table 3 shows the background, mean and threshold values of the trace metals in comparison with analytical results from other studies in order to obtain a preliminary inspection of the level of contamination in the bottom sediments. The median was chosen in this study as the local background because it is representative of the local data and less affected by outliers [79]. Threshold values were computed for the geochemical data subset following the elimination of outliers. The statistically derived threshold values are crucial in distinguishing between geogenic and anthropogenic sources of the trace metals [80]. These values represent the upper limit of the background concentrations of the potential toxic trace metals in sediments [10] and allows the identification of anomalous concentrations. Because stream sediments are an essential and dynamic component of catchments, they reflect the average chemical composition of the mixture of soils, sediments and rocks [32]. In this regard, the trace metal contents in this study were presented alongside local background levels in soils [49] and mean concentrations in ferralsols of the Sub-Saharan Africa region [75]. All trace metal concentrations (with corresponding data in local soils) in the Lom sediments were lower than the mean levels in local soils. About 18% of the sediment samples exceeded the concentrations of Fe and Mn in ferralsols. Based on this comparison, low concentration of trace metals in the sediment is likely due to the interplay of deep weathering, depleted parent rocks and the incorporation of metals in ferruginous clays or organic matter in the lateritic soil cover.
The degree of correlation between trace metals in the bottom sediments is given in Table 4. Interestingly, except for Fe and Mn, all trace metals correlated negatively with pH. The poor correlations suggest these metals are relatively immobile at near neutral pH, thus accounting for their low concentrations. Similarly, Fe and Mn correlated poorly with all other elements suggesting a less co-precipitation effect on them in this drainage system. Unlike Fe and Mn, strong positive correlations were observed between Co–Cr–Ni–As–Se–V–Pb and V–Cr–Co–Ni–As and likely indicate that they are sourced from the granitic rocks and partial dissolution of sulphides within the catchment. Indeed, the complete dissolution of metal sulphides is effective under acidic conditions rather than the near neutral pH (mean = 6.4, Table 2) of the sediments.
Factor analysis was applied on the log-transformed geochemical data to infer the controlling factors behind multi-element associations in relation to catchment geology, mineralization or anthropogenic activities. The spatial distribution of the factor scores of the element associations and their relationship with the geology are shown in Fig. 3a–c. Three factors explaining about 79% of the variance were generated (Table 5).
Factor 1 accounts for 36.5% of the total variability. This dipolar factor showed high positive loadings for Ni, Cr, V, Co, As, Se and a negative loading for pH. The F1 association showed high and medium factors scores relating to the metamorphic basement of the catchment (Fig. 3a). Sediments derived from granitic rocks and other felsic metasedimentary rocks such as quartzites and amphibolitic schists that make up the Lom basement are known to be poor in V, Cr, Ni, Co, and As [79.] Besides, Co, V and Ni can easily replace Fe in magnetite [81] which is a major oxide in the ferralitic soils of this tropical basin [82]. The presence of As in this factor is attributable to arsenopyrite dissemination in the parent rocks. Arsenopyrite has been reported as a separate sulphide mineralization event distinct from the main chalcopyrite sulphidation in the study area [66]. The negative contribution of pH in this factor implies that an acidic environment is required for these metals to be released from their geological materials.
A significant proportion of data variability (29.9%) described by Factor 2 is associated with scores of chalcophiles (Cu–Zn–Hg–Pb–Cd) and Sc. High factor scores (> 0) of these elements occur around reported gold indications reflecting sulphide gold-quartz vein mineralization (Fig. 3b). Moreover, previous studies have reported the occurrence of chalcopyrite (Cu), sphalerite (Zn) and galena (Pb) in the underlying rocks of the study area [45, 83]. The negative association of As and chalcophile elements is consistent with the claims that two distinct hydrothermal events are related to the epithermal gold mineralization in the study area [66]. Also, As and Pb have been identified as potential pathfinder elements for gold in the area. The contents and geochemical dispersion haloes of these metals in different lateritic profiles in the area were used to indicate gold mineralization. Arsenic was widely dispersed in soils and considered useful in regional survey while Pb was suited to follow up work [82]. Scandium is likely associated with organic matter. Its small size and high charge favour the formation of stable organic complexes in soils or adsorption on clay minerals derived from the chemical weathering of the granitic rocks [84].
The manganiferous relationship in factor 3 is a clear indication of the presence of Fe-bearing rocks and the co-precipitation effect. Accordingly, the highest F3 factor scores are located in the area underlain by the volcaniclastic schists and the metasedimentary rocks, quartzites and metaconglomerates (Fig. 3c). Iron and Mn exist as compensating ions on clay complexes and their precipitation is mainly dependent on the pH of the sediments in the catchment [85]. Hence, the poor correlation observed between Fe and Mn and the other trace metals suggests that they do not play a major role in scavenging these elements under near neutral conditions.
Spatial geochemical features
Spatial distributions of high levels of Co, Cr, V and Ni (Fig. 4a–d) cluster in the eastern part and correspond to areas underlain by upper gneisses and granodiorite (Fig. 2). Similarly, As distribution (Fig. 4e) is controlled by the catchment geology even though its concentrations were lower than the calculated threshold (Table 3). Moreover, relatively high concentrations of As coincided with some reported gold occurrences in the area. As previously stated, this observation is in line with the assertion that As is an important pathfinder for gold in this basin [82].
Contrary to As, the base metals Cu, Zn, Pb and Sc have very low values around gold deposits (Fig. 5a–d; for gold occurrence, see Fig. 2). No anomalous sites were observed for these metals and their background concentrations indicate sulphide mineralization related to vein gold deposits. Whole rock geochemistry of the gold-quartz veins by Vishiti et al. [48] suggests a generally low base metal content resulting from the reaction between the hydrothermal fluid and the granitic rocks in the area. This explains the very low concentration of the base metals in the bottom sediments.
Hot spots of Fe and Mn occur in the NW-SW portion of the study area (Fig. 6a, b). These elements are important constituents in ferromagnesian silicates and oxides in the underlying metamorphic rocks. In addition to the hypogene and supergene hematite sources, other possible sources of Fe in the studied sediments are the sulphide minerals pyrite and arsenopyrite associated with the primary gold bearing quartz veins [48]. Besides Cu, As and Pb, Fe is considered as another pathfinder for gold in the area [46]. Hence, deep chemical weathering in this tropical basin results in the enrichment of Fe and Mn in the sediments. In the light of environmental significance, Fe and Mn are environmental scavengers. Heavy metals such as Cu, Zn and Pb and the metalloid As can form stable complexes with Fe and Mn oxides through adsorption or co-precipitation processes [86]. Also, Fe and Mn oxides form thin coatings on minerals and clay particles which serve as natural traps or carriers of the heavy metals discharged into the aquatic system [87].
The distribution patterns of Cd, Hg and Se were distinct (Fig. 7a–c). Cadmium had lower concentrations (maximum concentration = 3.8 µg/kg) compared to the estimated threshold (13.21 µg/kg) and crustal average (98 ppb) [88]. Despite the dissimilar distribution trends for Se and Hg (Fig. 7b, c), Se:Hg ratios can be used to check for Hg contamination in sediments [89]. The Hg-to-Se molar ratio was first proposed by Ganther et al. [90] as a reference standard for Hg contamination. Ralston [91] later suggested that Se:Hg > 1 implies Se plays a key role in Hg assimilation processes. Using this approach, the ratios were calculated and presented in Table 2. Molar ratios above 1 indicate low Hg content and a protective effect of Hg toxicity, and vice versa. The estimated ratios ranged from 0.04 to 7.49. More than 80% of the samples had Se:Hg > 1 indicating that the sediments had higher Se contents over Hg. Thus, the negative effects of Hg are neutralized by the relatively higher Se content through assimilation processes. In the study area, gold amalgamation is practiced [92] and therefore, a plausible source of Hg in the sediments. Naturally, Hg occurs in trace amounts in the earth’s crust [93]. Through its use in mining, Hg may be discharged into water, deposited in sediments or released into the atmosphere. Mercury is a toxic substance which affects the reproductive and nervous system [94]. It is therefore important to monitor the use of the hazardous element in mining within the catchment (Fig. 7).
Conclusions
For the first time, the mineral composition, background values, threshold values and baseline environmental geochemical assessment of stream sediments from the lower Lom Basin have been made available. Mineralogically, quartz, phyllosilicates (muscovite + kaolinite) and feldspars constitute the dominant mineral phases in the sediments. These minerals are derived primarily from the weathering of the complex plutono-metamorphic basement and influenced by hydraulic energy and sorting. In terms of trace metals, concentrations of Sc, Cu, Zn, As, Se, Cd, Hg and Pb were low while V, Cr, Co, Ni, Mn and Fe were slightly enriched compared to their calculated threshold values. Overall, the low trace metal content of stream sediments is the result of the interaction of the near neutral pH of sediments (which does not favour the dissolution of metal sulphides), impoverished bedrocks and chemical weathering.
Multivariate statistical techniques enabled us to comprehend the basic processes influencing spatial geochemical variability. The spatial distribution of the trace metals Ni, Cr, V, Co and Se is controlled largely by source geology. Arsenic distribution showed a coherent relationship to the occurrence of Au deposits in some parts of the study area. Mercury, a hazardous environmental pollutant, is released into the basin through its use in gold recovery. Its continued use in refining gold may lead to harmful levels in the sediments.
The results obtained from this study show that the sediments have not been impacted by mining practices. However, given the paucity in fundamental geochemical data in Cameroon, this newly generated stream sediment data will serve as guidelines for future studies (environment, health and agriculture) in the region and other mineralized areas in the country. Future work should include the examination of metal composition in environmental samples from abandoned and active mine sites for comparison and environmental health risk assessment.
References
Laszlo O, Istvan H, Ubul F (1997) Low density geochemical mapping in Hungaria. J Geochem Explor 60:55–66
Rapant S, Raposova M, Bodisa D, Marsin K, Slaninka I (1999) Environmental–geochemical mapping program in the Slovak Republic. J Geochem Explor 66:151–158
Reimann C, Kashulina G, de Caritat P, Niskavaara H (2001) Multi-element, multi-medium regional geochemistry in the European arctic: element concentration, variation and correlation. Appl Geochem 16:759-780
Birke M, Rauch U, Stummeyer J (2015) How robust are geochemical patterns? A comparison of low and high sample density geochemical mapping in Germany. J Geochem Explor 154:105–128
de Caritat P, Cooper M (2016) A continental-scale geochemical atlas for resource exploration and environmental management: the national geochemical survey of Australia. Geochem Explor Environ Anal 16:3–13
de Caritat P, Lech ME, McPherson AA (2008) Geochemical mapping ‘down under’: selected results from pilot projects and strategy outline for the national geochemical survey of Australia. Geochem Explor Environ Anal 8:301–312
Chiprés JA, Salinas JC, Castro-Larragoitia J, Monroy MG (2008) Geochemical mapping of major and trace elements in soils from the Altiplano Potosino, Mexico: a multiscale comparison. Geochem Explor Environ Anal 8:279–290
Garret RG, Reimann C, Smith DB, Xie X (2008) From geochemical prospecting to international geochemical mapping: a historical overview. Geochem Explor Environ Anal 8:205–217
Smith DB, Reimann C (2008) Low-density geochemical mapping and the robustness of geochemical patterns. Geochem Explor Environ Anal 8:219–227
Rose AW, Hawkes HE, Webb JS (1979) Geochemistry in mineral exploration, 2nd edn. Academic Press, London
Webb JS, Thornton I, Thompson M, Howarth RJ, Lowenstein PL (1978) The Wolfson geochemical atlas of England and Wales. Clarendon Press, Oxford
Boboye OA, Abumere IO (2014) Environmental impact of elemental concentration and distribution in waters, soils and plants along the Lokoja-Abuja pipeline routes of Bida Basin, northwestern Nigeria. J Afr Earth Sci 99:694–704
Förstner U, Ahlf W, Calmano W, Kersten M (1991) Geochemistry of the Arno river sediments 545 sediment criteria development. In: Heling D, Rothe P, Förstner U, Stoffers P (eds) Sediments and environmental geochemistry. Springer, Berlin, pp 312–338
Howarth RJ, Thornton I (1983) Regional geochemical mapping and its application to environmental studies. In: Thorton I (ed) Applied environmental geochemistry. Academic Press, London, pp 41–73
Kim JY, Chon HT (2001) Pollution of a water course impacted by acid mine drainage in the Imgok creek of the Gangreung coal field, Korea. Appl Geochem 16:1387–1396
Likuku AS, Mmolawa KB, Gaboutloeloe GK (2013) Assessment of heavy metal enrichment and degree of contamination around the copper–nickel mine in the Selebi Phikwe Region, Eastern Botswana. Environ Ecol Res 1(2):32–40
Van Straaten P (2000) Mercury contamination associated with small-scale gold mining in Tanzania and Zimbabwe. Sci Total Environ 259:105–113
Hawkes HE, Webb JS (1962) Geochemistry in mineral exploration. Harper Collins, New York
Salminen R, Gregorauskiene V (2000) Considerations regarding the definition of a geochemical baseline of elements in the surficial materials in areas differing in basic geology. Appl Geochem 15(5):647–653
Gałuszka A (2007) A review of geochemical background concepts and an example using data from Poland. Environ Geol 52:861–870. https://doi.org/10.1007/s00254-006-0528-2
Reimann C, Garrett RG (2005) Geochemical background—concept and reality. Sci Total Environ 350:12–27
Guillén MT, Delgado J, Albanese S, Annamaria Lima JMN, De Vivo B (2011) Environmental geochemical mapping of Huelva municipality soils (SW Spain) as a tool to determine background and baseline values. J Geochem Explor 109:59–69
Matschullat J, Ottenstein R, Reimann C (2000) Geochemical background—can we calculate it? Environ Geol 39:990–1000. https://doi.org/10.1007/s002549900084
Gałuszka A, Migaszewski ZM (2011) Geochemical background—an environmental perspective. Mineralogia 42(1):7–17. https://doi.org/10.2478/v10002-011-0002-y
Dinelli E, Cortecci G, Lucchini F, Zantedeschi E (2005) Sources of major and trace elements in the stream sediments of the Arno river catchment (northern Tuscany, Italy). Geochem J 39:531–545
Lapworth DJ, Knights KV, Key RM, Johnson CC, Ayoade E, Adekanmi MA, Arisekola TM, Okunlola OA, Backman B, Eklund M, Everett PA, Lister RT, Ridgway J, Watts MJ, Kemp SJ, Pifield PEJ (2012) Geochemical mapping using stream sediments in west-central Nigeria: implications for environmental studies and mineral exploration in West Africa. Appl Geochem 27:1035–1052
Ohta A, Imai N, Terashima S, Tachibana Y, Ikehara K, Nakajima T (2004) Geochemical mapping in Hokuriku, Japan: influence of surface geology, mineral occurrences and mass movement from terrestrial to marine environments. Appl Geochem 19:1453–1469
Ohta A, Imai N, Terashima S, Tachibana Y (2011) Regional geochemical mapping in eastern Japan including the nation’s capital, Tokyo. Geochem Explor Environ Anal 11:211–223
Zhizhong C, Xuejing X, Wensheng Y, Jizhou F, Qin Z, Jindong F (2014) Multi-element geochemical mapping in Southern China. J Geochem Explor 139:183–192
Zuluaga MC, Norini G, Lima A, Albanese S, David CP, De Vivo B (2017) Stream sediment geochemical mapping of the Mount Pinatubo-Dizon Mine area, the Philippines: implications for mineral exploration and environmental risk. J Geochem Explor 175:18–35. https://doi.org/10.1016/j.gexplo.2016.12.012
Mikoshiba UM, Imai N, Terashima S, Tachibana Y, Okay T (2006) Geochemical mapping in northern Honshu, Japan. Appl Geochem 21:492–514
Smith DB, Smith SM, Horton JD (2013) History and evaluation of national-scale geochemical data set for the United States. Geosci Front 4:167–183
Chandrajith R, Dissanayake CB, Tobshall HJ (2001) Enrichment of high field strength elements in stream sediments of a granulite terrain in Sri Lanka—evidence for a mineralised belt. Chem Geol 175:259–271
Plant JA, Raiswell R (1983) Principles of environmental geochemistry. In: Thonton I (ed) Applied environmental geochemistry. Academic Press, London, pp 1–40
Agyarko K, Dartey E, Kuffour RAW, Sarkodie PA (2014) Assessment of trace elements levels in sediment and water in some artisanal and small-scale mining (ASM) localities in Ghana. Curr World Environ 9(1):07–16
Sierra C, Ruíz-Barzola O, Menéndez M, Demey JR, Vicente-Villardón JL (2017) Geochemical interactions study in surface river sediments at an artisanal mining area by means of Canonical (MANOVA)-Biplot. J Geochem Explor 175:72–81. https://doi.org/10.1016/j.gexplo.2017.01.002
Taiwo AM, Awomeso JA (2017) Assessment of trace metal concentration and health risk of artisanal gold mining activities in Ijeshaland, Osun State Nigeria—part 1. J Geochem Explor 177:1–10. https://doi.org/10.1016/j.gexplo.2017.01.009
Embui VF, Omang BO, Che VB, Nforba MT, Suh EC (2013) Gold grade variation and stream sediment geochemistry of the theVaimba-Lidi drainage system, northern Cameroon. Nat Sci 5(2A):282–290
Etame J, Tchameni Ngouabe EG, Ngon Ngon GF, Ntamak-Nida MJ, Suh CE, Bilong MGP (2013) Mineralogy and geochemistry of active stream sediments from the Kelle River drainage system (Pouma, Cameroon). Sci Technol Dév 14:35–47
Mimba ME, Nforba MT, Suh CE (2014) Geochemical dispersion of gold in stream sediments of the Paleoproterozoic Nyong Series, southern Cameroon. Sci Res 2(6):155–165
Ngambu AA, Kouankap Nono GD, Kouske PA, Wotchoko P, Takodjou WJD, Agyingyi CM, Anzah AR, Suh CE (2016) Geochemical investigation of stream sediments from the nlonako area; Littoral, Cameroon: implications for Au, Ag, Cu, Pb and Zn mineralization potentials. Int J Adv Geosci 4(2):104–112
Soh TL, Ganno S, Kouankap Nono GD, Ngnotue T, Kankeu B, Nzenti JP (2014) Stream sediment geochemical survey of Gouap-Nkollo prospect, southern Cameroon: implications for gold and LREE exploration. Am J Min Metall 2(1):8–16. https://doi.org/10.12691/ajmm-2-1-2
Stendal H, Toteu SF, Frei R, Penaye J, Njel UO, Bassahak J, Kankeu J, Nagko V, Hell JV (2006) Derivation of detrital rutile in the Yaounde region from the Neoproterozoic Pan-African belt in southern Cameroon (Central Africa). J Afr Earth Sci 44:443–458
Ateh KI, Suh CE, Shemang EM, Vishiti A, Tata E, Chombong NN (2017) New LA-ICP-MS U-Pb ages, Lu-Hf systematics and REE characterization of zircons from a granitic pluton in the Betare Oya gold district, SE Cameroon. J Geosci Geomat 5(6):267–283
Fon AN, Che VB, Suh CE (2012) Application of electrical resistivity and chargeability data on a GIS platform in delineating auriferous structures in a deeply weathered lateritic terrain, East Cameroon. Int J Geosci 3:960–971
Mboudou GMM, Fozao KF, Njoh OA, Agyingi CM (2017) Characterization of alluvial gold bearing sediments of Betare Oya District-East Cameroon, implication for gold exploration and recovery. Open J Geol 7:1724–1738
Omang BO, Suh CE, Lehmann B, Vishiti A, Chombong NN, Fon A, Egbe JA, Shemang EM (2015) Microchemical signature of alluvial gold from two contrasting terrains in Cameroon. J Afr Earth Sci 112:1–14
Vishiti A, Suh CE, Lehmann B, Shemang EM, Ngome NLJ, Nshanji NJ, Chinjo FE, Mongwe OY, Egbe AJ, Petersen S (2017) Mineral chemistry, bulk rock geochemistry, and S-isotope signature of lode-gold mineralization in the Bétaré Oya gold district, south-east Cameroon. Geol J. https://doi.org/10.1002/gj.3093
Manga VE, Neba GN, Suh CE (2017) Environmental geochemistry of mine tailings soils in the artisanal gold mining district of Bétaré-Oya, Cameroon. Environ Pollut 6(1):52–61. https://doi.org/10.5539/ep.v6n1p52
Tehna N, Nguene FD, Etame J, Medza Ekodo JM, Noa TS, Suh CE, Bilong P (2015) Impending pollution of Betare Oya opencast mining environment (Eastern Cameroon). J Environ Sci Eng 4:37–46
Rakotondrabe F, Ngoupayou JRN, Mfonka Z, Rasolomanana EH, Abolo AJN, Ako AA (2017) Water quality assessment in the Betare-Oya gold mining area (East-Cameroon): multivariate statistical analysis approach. Sci Total Environ 610–611:831–844. https://doi.org/10.1016/j.scitotenv.2017.08.080
Mimba ME, Ohba T, Nguemhe Fils SC, Wirmvem MJ, Bate Tibang EE, Nforba MT, Aka FT (2017) Regional hydrogeochemical mapping for environmental studies in the mineralized Lom Basin, East Cameroon: a pre-industrial mining survey. Hydrology 5(2):15–31. https://doi.org/10.11648/j.hyd.20170502.11
Mimba ME, Ohba T, Nguemhe Fils SC, Wirmvem MJ, Numanami N, Aka FT (2017) Seasonal hydrological inputs of major ions and trace metal composition of streams draining the mineralized Lom Basin, East Cameroon: basis for environmental studies. Earth Syst Environ 1:22. https://doi.org/10.1007/s41748-017-0026-6
De Wit MJ, Linol B (2015) Precambrian basement of the Congo Basin and its flanking terrains. In: de Wit MJ et al (eds) Geology and resource potential of the Congo Basin, regional geology reviews. Springer, Berlin, pp 19–37. https://doi.org/10.1007/978-3-642-29482-2_2
Toteu SF, Penaye J, Poudjom DY (2004) Geodynamic evolution of the Pan-African belt in Central Africa with special reference to Cameroon. Can J Earth Sci 41:73–85
Van Schmus WR, Oliveira EP, Da Silva Filho AF, Toteu SF, Penaye J, Guimaraes IP (2008) Proterozoic links between the Borborema Province NE Brazil, and the Central African Fold Belt. Geol Soc Lond Spec Publ 294(1):69–99. https://doi.org/10.1144/SP294.5
Castaing C, Feybesse JL, Thieblemont D, Triboulet C, Chevremont P (1994) Palaeogeographical reconstructions of the Pan-African/Brasiliano orogen: closure of an oceanic domain or intracontinental convergence between major blocks? Precambrian Res 67:327–344
Ngako V, Affaton P, Njonfang E (2008) Pan-African tectonics in northwestern Cameroon: implication for the history of western Gondwana. Gondwana Res 14:509–522
Kankeu B, Greiling RO, Nzenti JP, Bassahak J, Hell JV (2012) Strain partitioning along the Neoproterozoic Central Africa shear zone system: structures and magnetic fabrics (AMS) from the Meiganga area, Cameroon. Neues Jahrb Geol Paläontologie Abh 265:27–47
Ngako V, Affaton P, Nnange JM, Njanko Th (2003) Pan-African tectonic evolution in central and southern Cameroon: transpression and transtension during sinistral shear movements. J Afri Earth Sci 36:207–221
Toteu SF, Penaye J, Deloule E, Van Schmus WR, Tchameni R (2006) Diachronous evolution of volcano-sedimentary basins North of the Congo craton: insights from U-Pb ion microprobe dating of zircons from the Poli, Lom and Yaounde’ Groups (Cameroon). J Afr Earth Sci 44:428–442
Soba D, Michard A, Toteu SF, Norman DI, Penaye J, Ngako V, Nzenti JP, Dautel D (1991) Donnes géochronologiques nouvelles (Rb–Sr, U–Pb et Sm–Nd) sur la zone mobile pan-africaine de l’Est Cameroun: âge Protérozoïque supérieur de la série du Lom. Comptes Rendus l’Acad Sci 312:1453–1458
Toteu SF, Van Schmus RW, Penaye J, Michard A (2001) New U-Pb and Sm-Nd data from north central Cameroon and its bearing on the pre-pan-African history of Central Africa. Precambrian Res 108:45–73
Bakia M (2014) East Cameroon’s artisanal and small-scale mining bonanza: how long will it last? The futures of small-scale mining in sub-Saharan Africa. Futures 62:40–50. https://doi.org/10.1016/j.futures.2013.10.022
Djibril KN, Cliford TB, Pierre W, Alice M, Kuma CJ, Flore TD (2017) Artisanal gold mining in Batouri area, East Cameroon: impacts on the mining population and their environment. J Geol Min Res 9(1):1–8. https://doi.org/10.5897/JGMR16.0263
Omang BO, Bih CV, Fon NN, Suh CE (2014) Regional geochemical stream sediment survey for gold exploration in the upper Lom basin, eastern Cameroon. Int J Geosci 5:1012–1026
Salminen R, Tarvainen T, Demetriades A, Duris M, Fordyce FM, Gregorauskiene V, Kahelin H, Kivisilla J, Klaver G, Klein H, Larson JO, Lis J, Locutura J, Marsina K, Mjartanova H, Mouvet C, O’Connor P, Odor L, Ottonello G, Paukola T, Plant JA, Reimann C, Schermann O, Siewers U, Steenfelt A, Van der Sluys J, de Vivo B, Williams L (1998) FOREGS geochemical mapping field manual. Geol Surv Finl 1998(47):16–21
Makishima A, Nakamura E (1997) Suppression of matrix effects in ICP-MS by high power operation of ICP: application to precise determination of Rb, Sr, Y, Cs, Ba, REE, Pb, Th, and U at ng g-1 levels in milligram silicate samples. J Geostand Geoanal 21(2):307–319
International Soil Reference and Information Centre (ISRIC) (2002) Procedures for soil analysis, Technical Paper 9, 6th edn, pp 101
Reimann C, Filmoser P, Garrett RG (2005) Background and threshold: critical comparison of methods of determination. Sci Total Environ 346:1–16
Sobrinho EC, Ribeiro J, Neto C, Sant’Ovaia H, Rocha F, Flores D, de Carvalho CG (2014) Mineralogy and geochemistry of sediments from São Francisco stream (Amazonian River Basin, Brazil). Comunicações Geológicas 101(1):57–60
Silva MMVG, Cabral Pinto MMS, Carvalho PCS (2016) Major, trace and REE geochemistry of recent sediments from lower Catumbela River (Angola). J Afr Earth Sci 115:203–217. https://doi.org/10.1016/j.jafrearsci.2015.12.014
Zhang C, Manheim FT, Hinde J, Grossman JN (2005) Statistical characterization of large geochemical database and effect of sample size. Appl Geochem 20:1857–1874
Darwish MAG (2017) Stream sediment geochemical patterns around an ancient gold mine in the Wadi El Quleib area of the Allaqi region, south Eastern Desert of Egypt: implications for mineral exploration and environmental studies. J Geochem Explor 175:156–175. https://doi.org/10.1016/j.gexplo.2016.10.010
Towett EK, Shepherd KD, Tondoh JE, Winowiecki LA, Lulseged T, Nyambura M, Sila A, Vagen TG, Cadisch G (2015) Total elemental composition of soils in Sub-Saharan Africa and relationship with soil forming factors. Geoderma Reg 5:157–168
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. Taylor & Francis Group, Boca Raton, London, New York
Zhao G, He F, Dai X, Zhang S, Yu R (2014) Ultra-low density geochemical mapping in Zimbabwe. J Geochem Explor 144:552–571
Ashley RP. Geoenvironmental model for low-sulfide gold-quartz vein deposits. In: U.S. Geological Survey. Chapter K. 2002. p. 20. https://pubs.usgs.gov/of/2002/of02-195/OF02-195K.pdf Accessed 18 May 2017
Ohta A, Imai N, Terashima S, Tachibana Y (2005) Application of multi-element statistical analysis for regional geochemical mapping in Central Japan. Appl Geochem 20:1017–1037
Reimann C, de Caritat P (2017) Establishing geochemical variation nd threshold values for 59 elements in Australian surface soil. Sci Total Environ 578:633–648. https://doi.org/10.1016/j.scitotenv.2016.11.010
Surour AA, El-Kammar AA, Arafa EH, Korany HM (2003) Dahab Stream Sediments southeastern Sinai, Egypt: a potential source of gold, magnetite and zircon. J Geochem Explor 7(1):25–43
Freyssinet PH, Lecompte P, Edimo A (1989) Dispersion of gold base metals in the Mborguene lateritic profile, East Cameroon. J Geochem Explor 32:99–116
Bafon TE. (2011) Quartz veining, wall rock alteration and mineralization at the Belikobone Prospect, Eastern Cameroon. Master’s thesis, University of Buea 2012, Buea, 52
Yang XJ, Lin A, Li XL, Wu Y, Zhou W, Chen Z (2013) China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ Dev 8:131–136. https://doi.org/10.1016/j.envdev.2013.03.006
Nganje TN, Adamu CI, Ygbaja AN, Ebieme E, Sikakwe G (2011) Environmental contamination of trace elements in the vicinity of Okpara coal mine, Enugu, Southeastern Nigeria. Arab J Geosci 44:199–205
Gomez AA, Valenzuela JLG, Aguayo SS, Meza DF, Ramirez JH, Ochoa GO (2007) Chemical partitioning of sediment contamination by heavy metals in the San Pedro River, Sonora, Mexico. Chem Spec Bioavail 19:25–35
Galan E, Gomez-Ariza JL, Gonzalez I, Fernandez-Caliani JC, Morales E, Giraldez I (2003) Heavy metal portioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18:409–421
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell Scientific Publication, Oxford
Sakan S, Sakan N, Anđelkovićc I, Trifunovićd S, Đorđevića D (2017) Study of potential harmful elements (arsenic, mercury and selenium) in surface sediments from Serbian rivers and artificial lakes. J Geochem Explor 180:24–34. https://doi.org/10.1016/j.gexplo.2017.06.006
Ganther HE, Goudie C, Wagner P, Sunde ML, Kopecky MJ, Oh SH, Hoekstra WG (1972) Selenium relation to decreased toxicity of methylmercury added to diets containing tuna. Science 175:1122–1124
Ralston NVC (2008) Selenium health benefit values as seafood safety criteria. EcoHealth 5:442–455
CAPAM (2016) Mission de suivi environnemental des sites d’exploitations minières artisanales dans les régions de l’Est et de l’Adamaoua. Technical repport, pp 44
Cava MP, Rodenas TE, Morales RA, Cervera ML, Guardia MC (2004) Vapour atomic fluorescence determination of mercury in milk by slurry sampling using multi commutation. Anal Clin Acta 506:145–153
Frumkin H, Letz R, Williams PL, Gerr F, Pierce M, Sanders A, Elon L, Mannings CC, Woods JS, Hertzberg VS, Mueller P, Taylor BB (2001) Health effects of long term mercury exposure among chloralkali plants workers. Am J Ind Med 39:1–18
Toteu SF, Penaye J, Deschamps Y, Maldan F, Nyama Atibagoua B, Bouyo Houketchang M, Sep Nlomgan JP, Mbola Ndzana SP. Géologie et ressources minérales du Cameroun. In: 33rd International Geological Congress, Oslo, Norway. 2008
Authors’ contributions
Study conception and design: TO, FTA, CES and MEM. Acquisition of data: MEM, SCNF, MTN, NN, TGB. Analysis and interpretation: MEM, SCNF, MTN, NN, TGB. Drafting of manuscript: MEM. Critical revision: MEM, TO, FTA, CES, SCNF, MTN, NN, TGB. All authors read and approved the final manuscript.
Acknowledgements
This research is part of the Ph.D. thesis of MEM and it benefitted financially from the Japan Agency of Science and Technology (JST) through the Japanese Government (MONBUKAGAKUSHO) Scholarship under the Ministry of Education, Culture, Sports, Science and Technology (MEXT). The authors thank IRGM for providing transportation facilities. All the staff of the Laboratory of Inorganic Chemistry and Material Science in Tokai University are truly appreciated for performing the XRD analysis. We extend gratitude to Drs. Asobo Elvis, Vishiti Akumbom for their inspiring suggestions before submission and two anonymous reviewers for their valuable comments which improved the quality of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mimba, M.E., Ohba, T., Nguemhe Fils, S.C. et al. Regional geochemical baseline concentration of potentially toxic trace metals in the mineralized Lom Basin, East Cameroon: a tool for contamination assessment. Geochem Trans 19, 11 (2018). https://doi.org/10.1186/s12932-018-0056-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12932-018-0056-5