Abstract
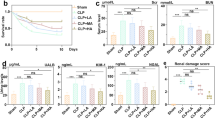
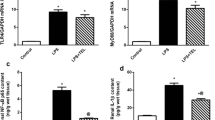
Blockade of indoleamine 2,3-dioxygenase (IDO) has been shown to alleviate lipopolysaccharide (LPS)-induced endotoxic shock and reduce sepsis mortality, but its effect on LPS-induced kidney damage has not been reported. Herein, we established a mouse kidney injury model by intraperitoneal injection of 10 mg/kg LPS and established an in vitro renal tubular epithelial cell injury model by stimulating TCMK-1 cells with 10 mg/L LPS. We found that pretreatment with 1-methyl tryptophan (1-MT), an IDO inhibitor, significantly improved LPS-induced mouse survival, and IDO knockout (KO) mice also had higher survival rates after LPS exposure than wild-type mice. At the same time, IDO KO or pretreatment with 1-MT not only reduced serum creatinine, blood urea nitrogen, renal tubular injury pathological score, but also inflammatory factors and oxidative stress status in serum or kidney of LPS-exposed mice. In vitro, blockade of IDO with 1-MT significantly inhibited LPS-induced apoptosis, inflammation and oxidative stress in TCMK-1 cells. In addition, blockade of IDO significantly inhibited LPS-activated TLR4/NF-κB signaling pathway in kidney of mice or in TCMK-1 cells. In conclusion, our results suggested that blockade of IDO attenuated kidney inflammation, apoptosis and oxidative stress to protect against LPS-induced septic kidney injury via inhibiting the TLR4/NF-κB signaling pathway.







Similar content being viewed by others
Data availability statement
The datasets generated and/or used during the present study are available from the corresponding author upon reasonable request.
References
Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:2050312119835043. https://doi.org/10.1177/2050312119835043.
Font MD, Thyagarajan B, Khanna AK. Sepsis and septic shock - Basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am. 2020;104(4):573–85. https://doi.org/10.1016/j.mcna.2020.02.011.
Prest J, Sathananthan M, Jeganathan N. Current trends in sepsis-related mortality in the United States. Crit Care Med. 2021;49(8):1276–84. https://doi.org/10.1097/ccm.0000000000005017.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global Burden of disease study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/s0140-6736(19)32989-7.
van Wagenberg L, Witteveen E, Wieske L, Horn J. Causes of mortality in ICU-Acquired weakness. J Intensive Care Med. 2020;35(3):293–6. https://doi.org/10.1177/0885066617745818.
Goulden R, Hoyle MC, Monis J, Railton D, Riley V, Martin P, et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35(6):345–9. https://doi.org/10.1136/emermed-2017-207120.
Majumdar A. Sepsis-induced acute kidney injury. Indian J Crit Care Med. 2010;14(1):14–21. https://doi.org/10.4103/0972-5229.63031.
Zhang Y, Wang L, Meng L, Cao G, Wu Y. Sirtuin 6 overexpression relieves sepsis-induced acute kidney injury by promoting autophagy. Cell Cycle. 2019;18(4):425–36. https://doi.org/10.1080/15384101.2019.1568746.
Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z, et al. Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci. 2020. https://doi.org/10.1016/j.lfs.2020.117791.
Jin YH, Li ZT, Chen H, Jiang XQ, Zhang YY, Wu F. Effect of dexmedetomidine on kidney injury in sepsis rats through TLR4/MyD88/NF-κB/iNOS signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):5020–5. https://doi.org/10.26355/eurrev_201906_18094.
Zhong Y, Wu S, Yang Y, Li GQ, Meng L, Zheng QY, et al. LIGHT aggravates sepsis-associated acute kidney injury via TLR4-MyD88-NF-κB pathway. J Cell Mol Med. 2020;24(20):11936–48. https://doi.org/10.1111/jcmm.15815.
Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–74. https://doi.org/10.1038/nri1457.
Schmidt SV, Schultze JL. New Insights into IDO biology in bacterial and viral infections. Front Immunol. 2014;5:384. https://doi.org/10.3389/fimmu.2014.00384.
Moon JS, Cheong NR, Yang SY, Kim IS, Chung HJ, Jeong YW, et al. Lipopolysaccharide-induced indoleamine 2,3-dioxygenase expression in the periodontal ligament. J Periodontal Res. 2013;48(6):733–9. https://doi.org/10.1111/jre.12063.
O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–22. https://doi.org/10.1038/sj.mp.4002148.
Zhang J, Yi S, Li Y, Xiao C, Liu C, Jiang W, et al. The antidepressant effects of asperosaponin VI are mediated by the suppression of microglial activation and reduction of TLR4/NF-κB-induced IDO expression. Psychopharmacology. 2020;237(8):2531–45. https://doi.org/10.1007/s00213-020-05553-5.
Bahraoui E, Serrero M, Planès R. HIV-1 Tat - TLR4/MD2 interaction drives the expression of IDO-1 in monocytes derived dendritic cells through NF-κB dependent pathway. Sci Rep. 2020;10(1):8177. https://doi.org/10.1038/s41598-020-64847-y.
Jung ID, Lee MG, Chang JH, Lee JS, Jeong YI, Lee CM, et al. Blockade of indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J Immunol. 2009;182(5):3146–54. https://doi.org/10.4049/jimmunol.0803104.
Hoshi M, Osawa Y, Ito H, Ohtaki H, Ando T, Takamatsu M, et al. Blockade of indoleamine 2,3-dioxygenase reduces mortality from peritonitis and sepsis in mice by regulating functions of CD11b+ peritoneal cells. Infect Immun. 2014;82(11):4487–95. https://doi.org/10.1128/iai.02113-14.
Zhang J, Tao J, Ling Y, Li F, Zhu X, Xu L, et al. Switch of NAD salvage to de novo biosynthesis sustains SIRT1-RelB-dependent inflammatory tolerance. Front Immunol. 2019;10:2358. https://doi.org/10.3389/fimmu.2019.02358.
Li J, Zhang Z, Wang L, Jiang L, Qin Z, Zhao Y, et al. Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-κB pathway. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.782660.
Xia S, Lin H, Liu H, Lu Z, Wang H, Fan S, et al. Honokiol attenuates sepsis-associated acute kidney injury via the inhibition of oxidative stress and inflammation. Inflammation. 2019;42(3):826–34. https://doi.org/10.1007/s10753-018-0937-x.
Ding Y, Zheng Y, Huang J, Peng W, Chen X, Kang X, et al. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int Immunopharmacol. 2019;71:336–49. https://doi.org/10.1016/j.intimp.2019.03.043.
Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother. 2020. https://doi.org/10.1016/j.biopha.2019.109772.
Stasi A, Intini A, Divella C, Franzin R, Montemurno E, Grandaliano G, et al. Emerging role of lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2017;32(1):24–31. https://doi.org/10.1093/ndt/gfw250.
Sun M, Li J, Mao L, Wu J, Deng Z, He M, et al. p53 Deacetylation alleviates sepsis-induced acute kidney injury by promoting autophagy. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.685523.
Benavente FM, Soto JA, Pizarro-Ortega MS, Bohmwald K, González PA, Bueno SM, et al. Contribution of IDO to human respiratory syncytial virus infection. J Leukoc Biol. 2019;106(4):933–42. https://doi.org/10.1002/jlb.4ru0219-051rr.
Li Q, Li X, Quan H, Wang Y, Qu G, Shen Z, et al. IL-10(-/-) Enhances DCs immunity against chlamydia psittaci infection via OX40L/NLRP3 and IDO/Treg pathways. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.645653.
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207. https://doi.org/10.1016/j.it.2016.01.002.
Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Sosman JA, et al. Immunosuppressive IDO in cancer: mechanisms of action, animal models, and targeting strategies. Front Immunol. 2020;11:1185. https://doi.org/10.3389/fimmu.2020.01185.
Cinar I, Sirin B, Aydin P, Toktay E, Cadirci E, Halici I, et al. Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sci. 2019;221:327–34. https://doi.org/10.1016/j.lfs.2019.02.039.
Xiong S, Hong Z, Huang LS, Tsukasaki Y, Nepal S, Di A, et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J Clin Invest. 2020;130(7):3684–98. https://doi.org/10.1172/jci136908.
Sodhi RK, Bansal Y, Singh R, Saroj P, Bhandari R, Kumar B, et al. IDO-1 inhibition protects against neuroinflammation, oxidative stress and mitochondrial dysfunction in 6-OHDA induced murine model of Parkinson’s disease. Neurotoxicology. 2021;84:184–97. https://doi.org/10.1016/j.neuro.2021.03.009.
Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. https://doi.org/10.3389/fimmu.2014.00461.
Chen J-Q, Szodoray P, Zeher M. Toll-Like receptor pathways in autoimmune diseases. Clin Rev Allergy Immunol. 2016;50(1):1–17. https://doi.org/10.1007/s12016-015-8473-z.
Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45(12):e66. https://doi.org/10.1038/emm.2013.97.
Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G, et al. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 2021;9(1):226. https://doi.org/10.1186/s40168-021-01107-9.
Mahanonda R, Sa-Ard-Iam N, Montreekachon P, Pimkhaokham A, Yongvanichit K, Fukuda MM, et al. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol. 2007;178(2):1151–7. https://doi.org/10.4049/jimmunol.178.2.1151.
Salazar F, Awuah D, Negm OH, Shakib F, Ghaemmaghami AM. The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs. Sci Rep. 2017;7:43337. https://doi.org/10.1038/srep43337.
Hemmati S, Sadeghi MA, Mohammad Jafari R, Yousefi-Manesh H, Dehpour AR. The antidepressant effects of GM-CSF are mediated by the reduction of TLR4/NF-ĸB-induced IDO expression. J Neuroinflammation. 2019;16(1):117. https://doi.org/10.1186/s12974-019-1509-1.
Funding
This work was supported by 2023 Dongguan Social Development Science and Technology Project (general project) (Grant/Award Number: 20231800904482).
Author information
Authors and Affiliations
Contributions
JY: conceptualization, methodology, writing—original draft. WW: performed the experiments, writing—original draft. WZ and ZL: analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10157_2023_2332_MOESM1_ESM.tif
Supplementary file1 IDO protein expression in different groups of kidney tissues. (A) Representative IDO protein immunohistochemical staining images in kidney of different group at 12 hours after LPS exposure. (B) IDO positive cells in kidney of different group were compared at 12 hours after LPS exposure. (C) Representative protein bands of IDO protein in kidney tissues of different group at 12 hours after LPS exposure. 10 mice in each group. *** P<0.001 vs Control group, and ### P<0.001 vs WT group. (TIF 1325 KB)
10157_2023_2332_MOESM2_ESM.tif
Supplementary file2 IDO protein expression in TCMK-1 cells after different treatment. (A) Expression of IDO protein in TCMK-1 cells stimulated with different concentrations of LPS for 8 hours using immunoblotting. (B) Expression of IDO protein in TCMK-1 cells stimulated with 10 mg/L LPS for different hours using immunoblotting. (C) TCMK-1 were stimulated with 10 mg/L LPS for 8 hours, and one replicate was added 1mM of 1-MT at 12 h before LPS onset. Cells were harvested to detect IDO protein expression using immunoblotting. 3 independent repetitions of each test. *** P<0.001 vs Solvent group (0 mg/L LPS or 10 mg/L LPS treat for 0 hours), and ### P<0.001 vs LPS group. (TIF 275 KB)
About this article
Cite this article
Wu, W., Zhong, W., Lin, Z. et al. Blockade of Indoleamine 2,3-Dioxygenase attenuates lipopolysaccharide-induced kidney injury by inhibiting TLR4/NF-κB signaling. Clin Exp Nephrol 27, 495–505 (2023). https://doi.org/10.1007/s10157-023-02332-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02332-2