Abstract
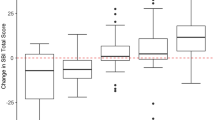
Patient quality of life (QOL) is an important metric of surgical success. To guide therapeutic advances in pituitary adenoma surgery, a validated, comprehensive instrument to quantify QOL is required. We aim to evaluate the validity of the 35 item anterior skull base questionnaire (ASBQ-35) in patients undergoing pituitary adenoma surgery. A total of 168 patients undergoing endoscopic resection of pituitary adenomas underwent longitudinal QOL assessment using the ASBQ-35 and the 22-item Sinonasal Outcomes Test (SNOT-22) over the first postoperative year. Validity of the ASBQ-35 was assessed by internal consistency, test–retest reliability, responsiveness to clinical change, and concurrent validity with the SNOT-22. Internal consistency of the ASBQ-35 was excellent, with a Cronbach’s alpha > 0.95 across all timepoints. Test–retest reliability between 3 and 6 months (ICC = 0.82, p < 0.001) and 6 months and 12 months (ICC = 0.78, p < 0.001) was robust. Concurrent validity with SNOT-22 was strong across all timepoints (absolute Pearson r ≥ 0.63, p < 0.001). Mean ASBQ-35 scores were significantly worse at 3 weeks compared to preoperative baseline (mean difference − 0.28, p < 0.01); however, by 12 months, scores had significantly improved (mean difference + 0.24, p < 0.01), indicating that the scale is responsive to clinical change. Each of the 6 domains of the ASBQ, and all 35 component questions, contributed to the discriminative of the ASBQ to measure QOL during the first postoperative year. The ASBQ-35 is a valid, comprehensive tool for assessing QOL after endoscopic pituitary adenoma surgery. Each component of the ASBQ-35 contributed to the overall assessment of QOL during the first postoperative year.
Similar content being viewed by others
Data availability
Available on reasonable request, in writing, to the corresponding author.
Code availability
Not applicable.
References
Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss DM (2004) Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg 100(5):813–819. https://doi.org/10.3171/jns.2004.100.5.0813
Gil Z, Abergel A, Spektor S et al (2003) Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol–head Neck Surg 129(12):1303–1309. https://doi.org/10.1001/archotol.129.12.1303
McCoul ED, Bedrosian JC, Akselrod O, Anand VK, Schwartz TH (2015) Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. JNS 123(3):813–820. https://doi.org/10.3171/2014.11.JNS14559
McCoul ED, Anand VK, Schwartz TH (2012) Improvements in site-specific quality of life 6 months after endoscopic anterior skull base surgery: a prospective study. J Neurosurg 117(3):498–506. https://doi.org/10.3171/2012.6.jns111066
Castle-Kirszbaum M, Wang YY, King J, Goldschlager T (2021) Quality of life following endoscopic surgical management of pituitary adenomas. Published online, Neurosurgery
Castle-Kirszbaum M, Uren B, Goldschlager T (2021) Anatomic variation for the endoscopic endonasal transsphenoidal approach. World Neurosurgery 156:111–119. https://doi.org/10.1016/j.wneu.2021.09.103
Kennedy JL, Hubbard MA, Huyett P, Patrie JT, Borish L, Payne SC (2013) Sino-nasal Outcome Test (SNOT-22): a predictor of post-surgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol 111(4):246-251.e2. https://doi.org/10.1016/j.anai.2013.06.033
Nunnally JC, Bernstein I (1994) Psychometric Theory. 3rd ed. McGraw Hill Higher Education. 007047849X
Higginson IJ, Carr AJ (2001) Using quality of life measures in the clinical setting. BMJ 322(7297):1297–1300
Webb SM, Badia X (2006) Validity and clinical applicability of the acromegaly quality of life questionnaire, AcroQoL: a 6-month prospective study. Eur J Endocrinol 155(2):269–277. https://doi.org/10.1530/eje.1.02214
Webb SM, Prieto L, Badia X et al (2002) Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clin Endocrinol (Oxf) 57(2):251–258. https://doi.org/10.1046/j.1365-2265.2002.01597.x
Rowles SV, Prieto L, Badia X, Shalet SM, Webb SM, Trainer PJ (2005) Quality of life (QOL) in patients with acromegaly is severely impaired: use of a novel measure of QOL: Acromegaly Quality of Life Questionnaire. J Clin Endocrinol Metab 90(6):3337–3341 (2020071619324226000)
Trainer PJ, Drake WM, Katznelson L et al (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342(16):1171–1177. https://doi.org/10.1056/NEJM200004203421604
van der Lely AJ, Gomez R, Pleil A et al (2017) Development of ACRODAT®, a new software medical device to assess disease activity in patients with acromegaly. Pituitary 20(6):692–701. https://doi.org/10.1007/s11102-017-0835-5
Webb SM, Badia X, Barahona MJ et al (2008) Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur J Endocrinol 158(5):623–630. https://doi.org/10.1530/EJE-07-0762
Santos A, Resmini E, Martínez-Momblán MA et al (2012) Psychometric performance of the CushingQoL questionnaire in conditions of real clinical practice. Eur J Endocrinol 167(3):337–342. https://doi.org/10.1530/EJE-12-0325
Milian M, Teufel P, Honegger J, Gallwitz B, Schnauder G, Psaras T (2012) The development of the Tuebingen Cushing’s disease quality of life inventory (Tuebingen CD-25). Part I: construction and psychometric properties. Clin Endocrinol (Oxf). 76(6):851–860. https://doi.org/10.1111/j.1365-2265.2011.04191.x
Milian M, Honegger J, Teufel P, Wolf A, Psaras T (2013) Tuebingen CD-25 is a sensitive tool to investigate health-related quality of life in Cushing’s disease patients in the course of the disease. Neuroendocrinology 98(3):188–199. https://doi.org/10.1159/000355622
Blum WF, Shavrikova EP, Edwards DJ et al (2003) Decreased quality of life in adult patients with growth hormone deficiency compared with general populations using the new, validated, self-weighted questionnaire, questions on life satisfaction hypopituitarism module. J Clin Endocrinol Metab. 88(9):4158–4167 (2020071616562423400)
Rosilio M, Blum WF, Edwards DJ et al (2004) Long-term improvement of quality of life during growth hormone (GH) replacement therapy in adults with GH deficiency, as measured by questions on life satisfaction-hypopituitarism (QLS-H). J Clin Endocrinol Metab. 89(4):1684–1693 (2016092613283600372)
McKenna SP, Doward LC, Alonso J et al (1999) The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res 8(4):373–383. https://doi.org/10.1023/a:1008987922774
Sarris CE, Little AS, Kshettry VR et al (2021) Assessment of the validity of the sinonasal outcomes test in pituitary surgery: a multicenter prospective trial. Laryngoscope. https://doi.org/10.1002/lary.29711. (Published online July 2021:lary.29711)
Little AS, Kelly D, Milligan J et al (2013) Prospective validation of a patient-reported nasal quality-of-life tool for endonasal skull base surgery: the Anterior Skull Base Nasal Inventory-12: Clinical article. JNS 119(4):1068–1074. https://doi.org/10.3171/2013.3.JNS122032
Little AS, Jahnke H, Nakaji P, Milligan J, Chapple K, White WL (2012) The anterior skull base nasal inventory (ASK nasal inventory): a clinical tool for evaluating rhinological outcomes after endonasal surgery for pituitary and cranial base lesions. Pituitary 15(4):513–517. https://doi.org/10.1007/s11102-011-0358-4
Ahmadipour Y, Müller O, Kreitschmann-Andermahr I, Mattheis S, Sure U (2020) Hütter BO (2000) Development, reliability, validity and sensitivity of the Sino-Nasal Outcome Test for Neurosurgery (SNOT-NC). Eur Arch Otorhinolaryngol 277(1):235–244. https://doi.org/10.1007/s00405-019-05661-9
Castle-Kirszbaum M, Kam J, Dixon B, Goldschlager T, King J, Wang YY (2021) Surgical outcomes and longitudinal quality of life after endoscopic endonasal surgery for anterior skull base meningioma. J Neurosurg 137:953–960
Castle-Kirszbaum M, Kam J, Wang YY, King J, Fryer K, Goldschlager T (2022) Surgical outcomes and quality of life in Rathke’s cleft cysts undergoing endoscopic transsphenoidal resection: a multicentre study and systematic review of the literature. Pituitary. https://doi.org/10.1007/s11102-021-01197-6. (Published online January 10, 2022)
Patel KS, Raza SM, McCoul ED et al (2015) Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg 123(3):571–580
Author information
Authors and Affiliations
Contributions
MCK: analysis of the data, writing the draft, revision. YYW: data collection, analysis of the data, revision. JK: data collection, analysis of the data, revision. JKam: data collection, analysis of the data, revision. MDYS: analysis of the data, revision. TG: data collection, analysis of the data, revision, study supervision.
Corresponding author
Ethics declarations
Ethics approval
HREC 15386Q (Institutional Approval).
Consent to participate
Not required.
Consent for publication
Not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castle-Kirszbaum, M., Wang, Y.Y., King, J. et al. Validation of the anterior skull base questionnaire 35 in endoscopic pituitary adenoma surgery. Neurosurg Rev 46, 7 (2023). https://doi.org/10.1007/s10143-022-01921-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-022-01921-1