Abstract
Background
The transnasal endoscopic approach to lesions of the skull base has come into routine practice in recent years. Standardized questionnaires to assess the postoperative sequelae are missing. The authors present a custom-made questionnaire for the sino-nasal outcome test for neurosurgery (SNOT-NC) in accordance with the SNOT-22.
Methods
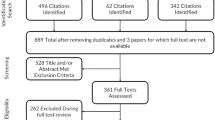
The SNOT-NC was developed with respect to the German version of the Sino-Nasal Outcome Test (SNOT-22) which is used for patients suffering from rhinosinusitis. It consists of 23 items covering “Nasal Discomfort”, Sleep Problems/Reduced Productivity”, “Ear and Head Discomfort”, “Visual Impairment” and “olfactory impairment”. The SNOT-NC was specifically adapted to patients undergoing transnasal operations of skull base lesions. The Short Form 36 health survey (SF-36), a nasal ad hoc questionnaire and the “Sniffin’ Sticks” test were used for analyzing convergent and divergent validity. The psychometric and clinimetric analyses were performed using the data of 102 consecutive patients treated by transnasal operations for skull base lesions.
Results
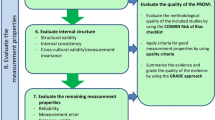
Factorial validity was secured by a confirmatory factor analysis. The internal consistency (Cronbach’s Alpha) for the subscales ranged from .62 to .85, while it was .84 for the whole instrument. The Guttman’s lower reliability limits range estimates corresponded closely to the Cronbach’s Alpha coefficients obtained. Examination of convergent and divergent validity revealed substantial associations between the SNOT-NC and a wide range of related nasal symptoms (p < .05). Different aspects of sensitivity were analyzed statistically with Cohen’s t statistic for change (pairwise t tests) exhibiting at least p < .05) underlining the sensitivity of the SNOT-NC.
Conclusions
The SNOT-NC appears to be a valid, reliable and sensitive measure for assessing the clinical outcome of patients undergoing transnasal endoscopic skull base surgery. The SNOT-NC proved to be a valuable tool to assess the nasal discomfort outcome of patients at follow-up examinations. Further analyses encompassing analyses for retest reliability are called for the future.
Similar content being viewed by others
References
Aaronson NK (1988) Quality of life: what is it? How should it be measured? Oncology (Williston Park, New York) 2:69–76
Baumann I, Blumenstock G, DeMaddalena H, Piccirillo JF, Plinkert PK (2007) Quality of life in patients with chronic rhinosinusitis: validation of the Sino-Nasal Outcome Test-20 German adapted version. HNO. https://doi.org/10.1007/s00106-005-1347-6
Beaton DE (2000) Understanding the relevance of measured change through studies of responsiveness. Spine (Phila Pa 1976). https://doi.org/10.1097/00007632-200012150-00015
Bullinger M, Kirchberger I (1998) SF-36 Fragebogen zum Gesundheitszustand. Diagnostische Verfahren der Rehabil. https://doi.org/10.1026//0084-5345.28.2.143
Cohen J (1988) Statistical power analysis for the behavioural sciences, 2nd edn. Lawrence Erlbaum Stat Power Anaylsis Behav Sci, New York
Costelo AB, Osborne J (2005) Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. https://doi.org/10.1234/2013/999990
Edwards D, Thawley SE, Haiduk A, Yonan C, Piccirillo JF (2007) Psychometric and clinimetric validity of the 31-Item Rhinosinusitis Outcome Measure (RSOM-31). Am J Rhinol. https://doi.org/10.2500/105065895781808711
Eid M, Gollwitzer M, Schmitt M (2013) Statistik und Forschungsmethoden: Lehrbuch, 3rd edn. Beltz, Weinheim, Basel
Erdfelder E, Faul F, Lang A-G, Buchner A (2007) GPOWER: a general power analysis program. Behav Res Methods. https://doi.org/10.3758/BF03193146
Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP (2009) Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. https://doi.org/10.1111/j.1749-4486.2009.01995.x
Hummel T, Konnerth CG, Rosenheim K, Kobal G (2001) Screening of olfactory function with a 4-min odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. https://doi.org/10.1177/000348940111001015
Husted JA, Cook RJ, Farewell VT, Gladman DD (2000) Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. https://doi.org/10.1016/S0895-4356(99)00206-1
Kaiser HF (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrika. https://doi.org/10.1007/BF02289233
Lienert GA, Raatz U (1998) Testaufbau und testanalyse. Ger Q. https://doi.org/10.2307/402200
Piccirillo JF, Merritt MG, Richards ML (2002) Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg. https://doi.org/10.1067/mhn.2002.121022
Rohrmann B (1978) Design and preliminary results of an interdisciplinary field study on urban noise. J Sound Vib. https://doi.org/10.1016/0022-460X(78)90485-6
Schlüter A, Ahmadipour Y, Vogelsang T et al (2016) Evaluation of the application of rhino-septal splints in endoscopic transsphenoidal skull base surgery. Eur Arch Oto Rhino Laryngol. https://doi.org/10.1007/s00405-016-4179-y
Siegel S, Castellan NJ (1988) Non-parametric statistics for the behavioural sciences. MacGraw Hill Int., New York
Streiner DL, Norman GR (2008) Health measurement scales: a practical guide to their development and use. Heal Meas Scales A Pract Guid to their Dev Use. https://doi.org/10.1093/acprof:oso/9780199231881.001.0001
Ware JE, Sherbourne CD (1992) The MOS 36-ltem Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. https://doi.org/10.1097/00005650-199206000-00002
Wright JG, Feinsten AR (1992) A comparative contrast of clinimetric and psychometric methods for constructing indexes and rating scales. J Clin Epidemiol. https://doi.org/10.1016/0895-4356(92)90161-F
Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD (1999) Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. https://doi.org/10.1097/00005650-199905000-00006
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was also approved by the local ethics committee (14-5791-BO).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix


Rights and permissions
About this article
Cite this article
Ahmadipour, Y., Müller, O., Kreitschmann-Andermahr, I. et al. Development, reliability, validity and sensitivity of the Sino-Nasal Outcome Test for Neurosurgery (SNOT-NC). Eur Arch Otorhinolaryngol 277, 235–244 (2020). https://doi.org/10.1007/s00405-019-05661-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05661-9