Abstract
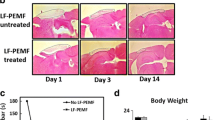
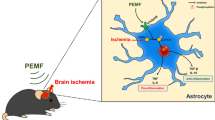
Pulsed electromagnetic fields (PEMF) have been demonstrated to have anti-inflammatory and pro-regenerative effects in animals and humans. We used the FDA-approved Sofpulse™ (Ivivi Health Sciences, LLC) to study effect of PEMF on infarct size and poststroke inflammation following distal middle cerebral artery occlusion (dMCAO) in mice. Electromagnetic field was applied within 30–45 min after ischemic brain damage and utilized twice a day for 21 consecutive days. Ischemic infarct size was assessed using MRI and histological analysis. At 21 days after dMCAO, the infarct size was significantly (by 26 %) smaller in PEMF-treated animals as compared to controls. Neuroinflammation in these animals was evaluated using specialized cytokine/chemokine PCR array. We demonstrate that PEMF significantly influenced expression profile of pro- and anti-inflammatory factors in the hemisphere ipsilateral to ischemic damage. Importantly, expression of gene encoding major pro-inflammatory cytokine IL-1α was significantly reduced, while expression of major anti-inflammatory IL-10 was significantly increased. PEMF application significantly downregulated genes encoding members of the major pro-apoptotic tumor necrosis factor (TNF) superfamily indicating that the treatment could have both anti-inflammatory and anti-apoptotic effects. Both reduction of infarct size and influence on neuroinflammation could have a potentially important positive impact on the poststroke recovery process, implicating PEMF as a possible adjunctive therapy for stroke patients.






Similar content being viewed by others
References
American Heart Association http://www.americanheart.org/presenter.jhtml?identifier=4725. 2010.
Statistics NY-PD http://nyp.org/health/disability-statistics.html. 2010.
Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111(6):483–95. doi:10.1016/j.clineuro.2009.04.001.
Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 Suppl 1:S232–40. doi:10.1038/sj.bjp.0706400.
Perera MN, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, et al. Inflammation following stroke. J Clin Neurosci. 2006;13(1):1–8. doi:10.1016/J.Jocn.2005.07.005.
Liguz-Lecznar M, Kossut M. Influence of inflammation on poststroke plasticity. Neural Plast. 2013;2013:258582. doi:10.1155/2013/258582.
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi:10.1186/1479-5876-7-97.
Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011;116(6):947–56. doi:10.1111/j.1471-4159.2010.07168.x.
Pilla AA. Nonthermal electromagnetic fields: from first messenger to therapeutic applications. Electromagn Biol Med. 2013;32(2):123–36. doi:10.3109/15368378.2013.776335.
Grant G, Cadossi R, Steinberg G. Protection against focal cerebral ischemia following exposure to a pulsed electromagnetic field. Bioelectromagnetics. 1994;15(3):205–16.
Sandyk R. Alzheimer's disease: improvement of visual memory and visuoconstructive performance by treatment with picotesla range magnetic fields. Int J Neurosci. 1994;76(3–4):185–225.
Sandyk R. Brief communication: electromagnetic fields improve visuospatial performance and reverse agraphia in a Parkinsonian patient. Int J Neurosci. 1996;87(3–4):209–17.
Mizushima Y, Akaoka I, Nishida Y. Effects of magnetic field on inflammation. Experientia. 1975;31(12):1411–2.
Rasouli J, Lekhraj R, White NM, Flamm ES, Pilla AA, Strauch B, et al. Attenuation of interleukin-1beta by pulsed electromagnetic fields after traumatic brain injury. Neurosci Lett. 2012;519(1):4–8. doi:10.1016/j.neulet.2012.03.089.
Rohde C, Chiang A, Adipoju O, Casper D, Pilla AA. Effects of pulsed electromagnetic fields on interleukin-1 beta and postoperative pain: a double-blind, placebo-controlled, pilot study in breast reduction patients. Plast Reconstr Surg. 2010;125(6):1620–9.
Heden P, Pilla AA. Effects of pulsed electromagnetic fields on postoperative pain: a double-blind randomized pilot study in breast augmentation patients. Aesthet Plast Surg. 2008;32(4):660–6. doi:10.1007/s00266-008-9169-z.
Nelson FR, Zvirbulis R, Pilla AA. Noninvasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: a randomized double-blind pilot study. Rheumatol Int. 2013;33(8):2169–73. doi:10.1007/s00296-012-2366-8.
Kuraoka M, Furuta T, Matsuwaki T, Omatsu T, Ishii Y, Kyuwa S, et al. Direct experimental occlusion of the distal middle cerebral artery induces high reproducibility of brain ischemia in mice. Exp Anim. 2009;58(1):19–29.
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–3. doi:10.1038/jcbfm.1990.47.
Jones PB, Shin HK, Boas DA, Hyman BT, Moskowitz MA, Ayata C, et al. Simultaneous multispectral reflectance imaging and laser speckle flowmetry of cerebral blood flow and oxygen metabolism in focal cerebral ischemia. J Biomed Opt. 2008;13(4):044007. doi:10.1117/1.2950312.
Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2(3):396–409. doi:10.1602/neurorx.2.3.396.
Wiessner C, Allegrini PR, Ekatodramis D, Jewell UR, Stallmach T, Gassmann M. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J Cereb Blood Flow Metab. 2001;21(7):857–64. doi:10.1097/00004647-200107000-00011.
Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134(6):1023–34. doi:10.1242/dev.000166.
Wojno ED, Hunter CA. New directions in the basic and translational biology of interleukin-27. Trends Immunol. 2012;33(2):91–7. doi:10.1016/j.it.2011.11.003.
Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. doi:10.1016/B978-0-12-394299-9.00001-1.
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–24. doi:10.1038/nn1715.
Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, et al. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern-Fetal Neonatal Med. 2007;20(11):777–92. doi:10.1080/14767050701483298.
Pan W, Kastin AJ. Tumor necrosis factor and stroke: role of the blood–brain barrier. Prog Neurobiol. 2007;83(6):363–74. doi:10.1016/j.pneurobio.2007.07.008.
Slevin M, Krupinski J, Mitsios N, Perikleous C, Cuadrado E, Montaner J, et al. Leukaemia inhibitory factor is over-expressed by ischaemic brain tissue concomitant with reduced plasma expression following acute stroke. Eur J Neurol. 2008;15(1):25–33. doi:10.1111/J.1468-1331.2007.01995.X.
Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1(alpha) and IL-1(beta) in ischemic brain damage (vol 21, pg 5528, 2001). J Neurosci. 2001;21(17):U11–U.
Ellison JA, Velier JJ, Spera P, Jonak ZL, Wang XK, Barone FC, et al. Osteopontin and its integrin receptor alpha(V)beta(3) are upregulated during formation of the glial scar after focal stroke. Stroke. 1998;29(8):1698–706.
Wang X, Louden C, Yue TL, Ellison JA, Barone FC, Solleveld HA, et al. Delayed expression of osteopontin after focal stroke in the rat. J Neurosci. 1998;18(6):2075–83.
Rutz S, Eidenschenk C, Ouyang WJ. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–32. doi:10.1111/Imr.12027.
Song W, Huo T, Guo F, Wang H, Wei H, Yang Q, et al. Globular adiponectin elicits neuroprotection by inhibiting NADPH oxidase-mediated oxidative damage in ischemic stroke. Neuroscience. 2013;248C:136–44. doi:10.1016/j.neuroscience.2013.05.063.
Muhl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3(9):1247–55. doi:10.1016/S1567-5769(03)00131-0.
Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14(5):409–26.
Watson ML, White AM, Campbell EM, Smith AW, Uddin J, Yoshimura T, et al. Anti-inflammatory actions of interleukin-13: suppression of tumor necrosis factor-alpha and antigen-induced leukocyte accumulation in the guinea pig lung. Am J Respir Cell Mol Biol. 1999;20(5):1007–12. doi:10.1165/ajrcmb.20.5.3540.
Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–72.
Banchereau J, Pascual V, O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–31. doi:10.1038/ni.2406.
Cuneo AA, Autieri MV. Expression and function of anti-inflammatory interleukins: the other side of the vascular response to injury. Curr Vasc Pharmacol. 2009;7(3):267–76.
Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi:10.1146/annurev.iy.10.040192.002211.
Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26.
Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8. doi:10.1186/Ar1917.
Beatus P, Jhaveri DJ, Walker TL, Lucas PG, Rietze RL, Cooper HM, et al. Oncostatin M regulates neural precursor activity in the adult brain. Dev Neurobiol. 2011;71(7):619–33. doi:10.1002/Dneu.20871.
Pilla AA. Electromagnetic fields instantaneously modulate nitric oxide signaling in challenged biological systems. Biochem Biophys Res Commun. 2012;426(3):330–3. doi:10.1016/j.bbrc.2012.08.078.
George I, Geddis MS, Lill Z, Lin H, Gomez T, Blank M, et al. Myocardial function improved by electromagnetic field induction of stress protein hsp70. J Cell Physiol. 2008;216(3):816–23. doi:10.1002/jcp.21461.
Pesce M, Patruno A, Speranza L, Reale M. Extremely low frequency electromagnetic field and wound healing: implication of cytokines as biological mediators. Eur Cytokine Netw. 2013;24(1):1–10. doi:10.1684/ecn.2013.0332.
Acknowledgments
This work was supported in part by Ivivi Health Sciences, LLC. We would like to thank Dr. Arthur Pilla for his valuable input and assistance. Microscopy images were generated in the UNM Cancer Center Fluorescence Microscopy Facility supported as detailed on the webpage http://hsc.unm.edu/crtc/microscopy/instru.html. Gene and miRNA analysis was performed in the Keck-UNM Genomics Resource (KUGR) facility and in Qiagen Company.
Conflict of Interest
Juan Carlos Pena-Philippides has no financial interest with the sponsor of the research. Yirong Yang has no financial interest with the sponsor of the research. Olga Bragina has no financial interest with the sponsor of the research. Sean Hagberg has financial interests with Ivivi Health Sciences. Edwin Nemoto has no financial interest with the sponsor of the research. Tamara Roitbak has no financial interest with the sponsor of the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pena-Philippides, J.C., Yang, Y., Bragina, O. et al. Effect of Pulsed Electromagnetic Field (PEMF) on Infarct Size and Inflammation After Cerebral Ischemia in Mice. Transl. Stroke Res. 5, 491–500 (2014). https://doi.org/10.1007/s12975-014-0334-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-014-0334-1