Abstract
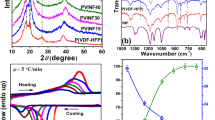
As people possess more safety conscious, the issue of electrolytes is attracting concern. The gel polymer electrolyte offers high ionic conductivity, wettability, and good interfacial contact, effectively reducing electrolyte leakage and improving battery safety. This study presents a novel gel polymer electrolyte that has been investigated for its excellent physical and electrochemical properties. The electrospinning film possesses a thin and flat surface with a thickness of only 40 ± 5 μm and particle size of around 300–400 nm. This creates an ion-conductive dual-channels that optimize the migration of lithium ions and ensure good contact and interfacial stability. In addition, the electrochemical stability window ranges from 0 ~ 6.1 V (vs. Li+/Li), which can be applied to most high-voltage cathode materials on the market. The NCM811//Li cell constructed with gel polymer electrolyte exhibited a first discharge capacity of 202.9 mAh g−1 at 0.5C and still maintain a discharge capacity of 117.5 mAh g−1 after 300 cycles, as well as a capacity retention rate of 57.9%. This strategy provides a new idea for the next generation of high-performance lithium metal batteries.





Similar content being viewed by others
Data availability
The data supporting this study’s findings are available from the corresponding authors on reasonable request.
References
Kong L, Li Y, Feng W (2021) Strategies to solve lithium battery thermal runaway: from mechanism to modification[J]. Electrochem Energy Rev 4(4):633–679
Xi C, Cui X, Zhang R et al (2022) Utilizing an oxygen-rich interface by hydroxyapatite to regulate the linear diffusion for the stable solid-state electrolytes[J]. ACS Appl Mater Interfaces 14(29):33392–33399
Poudyal R, Loskot P, Nepal R et al (2019) Mitigating the current energy crisis in Nepal with renewable energy sources[J]. Renew Sustain Energy Rev 116:109388
Xu G, Li R, Li M et al (2022) Rapid internal conversion harvested in Co/Mo dichalcogenides hollow nanocages of polysulfides for stable lithium-sulfur batteries[J]. Chem Eng J 434:134498
Wu X, Feng X, Yuan J et al (2022) Thiophene functionalized porphyrin complexes as novel bipolar organic cathodes with high energy density and long cycle life[J]. Energy Stor Mater 46:252–258
Li R, Li M, Chao Y et al (2022) Hexaoxacyclooctadecane induced interfacial engineering to achieve dendrite-free Zn ion batteries[J]. Energy Stor Mater 46:605–612
Xing J, Bliznakov S, Bonville L et al (2022) A review of nonaqueous electrolytes, binders, and separators for lithium-ion batteries[J]. Electrochem Energy Rev 5(4):14
Guo J, Chen Y, Xiao Y et al (2021) Flame-retardant composite gel polymer electrolyte with a dual acceleration conduction mechanism for lithium ion batteries[J]. Chem Eng J 422:130526
Cao G, Duan R, Li X et al (2022) Controllable catalysis behavior for high performance lithium sulfur batteries: from kinetics to strategies[J]. EnergyChem :100096
Chen D, Zhu M, Kang P et al (2022) Self-enhancing gel polymer electrolyte by in situ construction for enabling safe lithium metal battery[J]. Adv Sci 9(4):2103663
Tomaszewska A, Chu Z, Feng X et al (2019) Lithium-ion battery fast charging: a review[J]. ETransportation 1:100011
Shi Y, Yin P, Li J, et al (2023) Ultra-high rate capability of in-situ anchoring FeF3 cathode onto double-enhanced conductive Fe/graphitic carbon for high energy density lithium-ion batteries[J]. Nano Energy :108181.
Yang C, Shao R, Wang Q et al (2021) Bulk and surface degradation in layered Ni-rich cathode for Li ions batteries: defect proliferation via chain reaction mechanism[J]. Energy Stor Mater 35:62–69
Wang Z, Ni J, Li L (2022) Gradient designs for efficient sodium batteries[J]. ACS Energy Lett 7(11):4106–4117
York M, Larson K, Harris KC et al (2022) Recent advances in solid-state beyond lithium batteries[J]. J Solid State Electrochem 26(9):1851–1869
Dias FB, Plomp L, Veldhuis JB (2000) Trends in polymer electrolytes for secondary lithium batteries[J]. J Power Sources 88(2):169–191
Cheng X, Pan J, Zhao Y et al (2018) Gel polymer electrolytes for electrochemical energy storage[J]. Adv Energy Mater 8(7):1702184
Ahn JH, You T-S, Lee S-M et al (2020) Hybrid separator containing reactive, nanostructured alumina promoting in-situ gel electrolyte formation for lithium-ion batteries with good cycling stability and enhanced safety[J]. J Power Sources 472:228519
Li S, Liu J, Ma L et al (2022) Okra-like multichannel TiO@ NC fibers membrane with spatial and chemical restriction on shuttle-effect for lithium–sulfur batteries[J]. Adv Fiber Mater 1–14
Wang J, Wang Z, Ni J et al (2022) Electrospun materials for batteries moving beyond lithium-ion technologies[J]. Electrochem Energy Rev 5(2):211–241
Fan Z, Zi-Xuan X, Ming-Hu W (2022) Fault diagnosis method for lithium-ion batteries in electric vehicles using generalized dimensionless indicator and local outlier factor[J]. J Energy Storage 52:104963
Xi C, Xiao Y, Yang C et al (2023) Localized gelation cellulose separators enables dendrite-free anodes for the future-oriented zinc ion battery[J]. J Mater Chem A
Acknowledgements
This work was supported primarily by the National Natural Science Foundation of China (No. 22109025), National Key Research and Development Program of China (2020YFA0710303), and Natural Science Foundation of Fujian Province, China (2021J05121).
Author information
Authors and Affiliations
Contributions
Chengkai Yang: conceptualization, methodology, project administration, and funding acquisition. Ji Li and Xiancai Cui: investigation, methodology, data curation, and writing—original draft. Qilang Lin, Xiaolin Lyu, Qian Wang, and Yan Yu: mechanism discussion and funding acquisition. All authors discussed the results and commented on the manuscripts.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Cui, X., Lin, Q. et al. Construction of ion-conductive dual-channels by P(EA-co-AALi)-based gel electrolytes for high-performance lithium metal batteries. J Solid State Electrochem 27, 1383–1389 (2023). https://doi.org/10.1007/s10008-023-05492-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05492-z