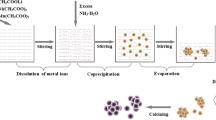
Abstract
At present, α-NaFeO2 lithium-rich layered oxides (LLO) as cathode materials for lithium-ion batteries have attracted widespread attention due to their structure and performance characteristics and have become the mainstream research materials for lithium-ion batteries. However, during the charge and discharge process, the irreversible phase transition, cation mixing, and oxygen loss on the surface will lead to the inevitable severe capacity attenuation. Aiming at these problems, researchers have carried out a lot of modification on the material to improve the electrochemical performances without changing the crystal structure. Element doping is one of the commonly used effective strategies. In this work, the recent progress in understanding the influences of dopants in LLO cathode materials were summarized through five types: dopants substituted for transition metal (TM), lithium, oxygen, respectively, and multiple-dopants, the element doping combined with other strategies. In addition, the development trend of element doping in LLO cathode materials was prospected. It is believed that this review can guide researchers on developing ion doping strategies for the LLO cathode materials.











Similar content being viewed by others
References
Wunderling N, Willeit M, Donges JF, Winkelmann R (2020) Global warming due to loss of large ice masses and Arctic summer sea ice. Nat Commun 11:1–8. https://www.nature.com/articles/s41467-020-18934-3
Kim S, Lee JE, Kim D (2019) Searching for the next new energy in energy transition: comparing the impacts of economic incentives on local acceptance of fossil fuels, renewable, and nuclear energies. Sustainability 11:2037. https://doi.org/10.3390/su11072037
Martins F, Felgueiras C, Smitkova M, Caetano N (2019) Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 12:964. https://doi.org/10.3390/en12060964
Giulia P, Maria R, Federico B, Raffaele C, Antonio P, Claudio G (2020) Poly(glycidyl ether)s recycling from industrial waste and feasibility study of reuse as electrolytes in sodium-based batteries. Chem Eng J 382:122934. https://doi.org/10.1016/j.cej.2019.122934
Marisa F, Cataldo S, Chiara F, Jijeesh RN, Giuseppina M, Federico B, Isabella N, Piercarlo M, Martin W, Claudio G (2019) Understanding the effect of UV-induced crosslinking on the physico-chemical properties of highly performing PEO/LiTFSI-based polymer electrolytes. Langmuir 35(25):8210–8219. https://doi.org/10.1021/acs.langmuir.9b00041
Li H, Wang Y, Wei Z, Yang X, Liang L, Xia L, Long M, Li Z, Zhang T (2022) Tunable copper complexes with functional ligands: a promising strategy for green primary explosives. Chem Eng J 430:132739. https://doi.org/10.1016/j.cej.2021.132739
Mao B, Liu C, Yang K, Li S, Liu P, Zhang M, Meng X, Gao, Duan Q, Wang Q, Sun J (2021) Thermal runaway and fire behaviors of a 300 Ah lithium ion battery with LiFePO4 as cathode. Renew Sustain Energy Rev 139:110717.https://doi.org/10.1016/j.rser.2021.110717
Samuel B, Timo J (2022) Sulfurized-polyacrylonitrile in lithium-sulfur batteries: Interactions between undercoordinated carbons and polymer structure under low lithiation. J Energy Chem 66:587–596. https://doi.org/10.1016/j.jechem.2021.08.070
Federico B, Stefano DL, Lucia F, Daniele V, Julia A, Carlotta F, Silvia B (2021) An overview on anodes for magnesium batteries: challenges towards a promising storage solution for renewables. Nanomaterials 11:810. https://doi.org/10.3390/nano11030810
Julia A, Claudia T, Daniele V, Davide D, Andrea M, Fabrizio C, Federico B, Carlotta F, Silvia B (2021) Nanosponge-based composite gel polymer electrolyte for safer Li-O2 batteries. Polymers 13:1625. https://doi.org/10.3390/polym13101625
Nurul NMR, Sharina AH, Azizan A, Nur HH, Federico B (2015) Effect of lithium bis(trifluoromethylsulfonyl)imide salt-doped UV-cured glycidyl methacrylate. J Solid State Electrochem 19:3079–3085. https://doi.org/10.1007/s10008-015-2910-z
Arianna M, Ana BM-G, Pasqualino M, Federico B, Giuseppina M, Claudio G, Michele P (2020) First-principles study of Na insertion at TiO2 anatase surfaces: new hints for Na-ion battery design. Nanoscale Adv 2:2745–2751. https://doi.org/10.1039/D0NA00230E
Mishra A, Mehta A, Basu S, Malode SJ, Shetti NP, Shukla SS, Nadagouda MN, Aminabhavi TM (2018) Electrode materials for lithium-ion batteries. Mater Sci Energy Technol 1:182–187. https://doi.org/10.1016/j.mset.2018.08.001
Hu M, Pang X, Zhou Z (2013) Recent progress in high-voltage lithium ion batteries. J Power Sources 237:229–242. https://doi.org/10.1016/j.jpowsour.2013.03.024
Wu F, Maier J, Yu Y (2020) Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem Soc Rev 49:1569–1614. https://doi.org/10.1039/C7CS00863E
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energ Environ Sci 4:3243–3262. https://doi.org/10.1039/C1EE01598B
Nayak PK, Grinblat J, Levi M, Wu Y, Powell B, Aurbach D (2014) TEM and Raman spectroscopy evidence of layered to spinel phase transformation in layered LiNi1/3Mn1/3Co1/3O2 upon cycling to higher voltages. J Electroanal Chem 733:6–19. https://doi.org/10.1016/j.jelechem.2014.09.005
Ahaliabadeh Z, Kong X, Fedorovskaya E, Kallio T (2022) Extensive comparison of doping and coating strategies for Ni-rich positive electrode materials. J Power Sources 540:231633. https://doi.org/10.1016/j.jpowsour.2022.231633
Yin S, Chen H, Chen J, Massoudi A, Deng W, Gao X, Zhang S, Wang Y, Lin T-W, Banks CE, Qiao S-Z, Zou G, Hou H, Ji X (2022) Chemical-mechanical effects in Ni-rich cathode materials. Chem Mater 34(4):1509–1523. https://doi.org/10.1021/acs.chemmater.1c03051
Park N-Y, Park G-T, Kim S-B, Jung W, Park B-C, Sun Y-K (2022) Degradation mechanism of Ni-rich cathode materials: focusing on particle interior. ACS Energy Lett 7(7):2362–2369. https://doi.org/10.1021/acsenergylett.2c01272
Pimenta V, Sathiya M, Batuk D, Abakumov AM, Giaume D, Cassaignon S, Larcher D, Tarascon J-M (2017) Synthesis of Li-rich NMC: a comprehensive study. Chem Mater 29(23):9923–9936. https://doi.org/10.1021/acs.chemmater.7b03230
Li M, Lu J (2020) Cobalt in lithium-ion batteries. Science 367(6481):979–980. https://doi.org/10.1126/science.aba9168
Toprakci O, Toprakci HAK, Li Y, Ji LW, Xue LG, Lee H, Zhang S, Zhang XW (2013) Synthesis and characterization of xLi2MnO3·(1–x)LiMn1/3Ni1/3Co1/3O2 composite cathode materials for rechargeable lithium-ion batteries. J Power Sources 241:522–528. https://doi.org/10.1016/j.jpowsour.2013.04.155
Liu JL, Chen L, Hou MY, Wang F, Che RC, Xia YY (2012) General synthesis of xLi2MnO3·(1–x)LiMn1/3Ni1/3Co1/3O2 nanomaterials by a molten-salt method: towards a high capacity and high power cathode for rechargeable lithium batteries. J Mater Chem 22:25380–25387. https://doi.org/10.1039/C2JM35026B
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J Mater Chem 15:2257–2267. https://doi.org/10.1039/B417616M
Gu M, Belharouak I, Zheng J, Wu H, Xiao J, Genc A, Amine K, Thevuthasan S, Baer DR, Zhang JG, Browning ND, Liu J, Wang C (2013) Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 7:760–767. https://doi.org/10.1021/nn305065u
Li Q, Ning D, Wong D, An K, Tang Y, Zhou D, Schuck G, Chen Z, Zhang N, Liu X (2022) Improving the oxygen redox reversibility of Li-rich battery cathode materials via Coulombic repulsive interactions strategy. Nat Commun 13:1123. https://www.nature.com/articles/s41467-022-28793-9
Zheng F, Yang C, Xiong X, Xiong J, Hu R, Chen Y, Liu M (2015) Nanoscale surface modification of lithium-rich layered-oxide composite cathodes for suppressing voltage fade. Angew Chem Int Edit 54:13058–13062. https://doi.org/10.1002/anie.201506408
Fu F, Wang Q, Deng YP, Shen CH, Peng XX, Huang L, Sun SG (2015) Effect of synthetic routes on the rate performance of Li-rich layered Li1.2Mn0.56Ni0.12Co0.12O2. J Mater Chem A 3:5197–5203. https://doi.org/10.1039/C4TA06552B
Armstrong AR, Holzapfel M, Novak P, Johnson CS, Kang SH, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J Am Chem Soc 128:8694–8698. https://doi.org/10.1021/ja062027
Jiang M, Key B, Meng YS, Grey CP (2009) Electrochemical and structural study of the layered, “Li-excess” lithium-ion battery electrode material Li[Li1/9Ni1/3Mn5/9]O2. Chem Mater 21:2733–2745. https://doi.org/10.1021/cm900279u
Gao J, Manthiram A (2009) Eliminating the irreversible capacity loss of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode by blending with other lithium insertion hosts. J Power Sources 191:644–647. https://doi.org/10.1016/j.jpowsour.2009.02.005
Liu Y, Yang Z, Zhong J, Li J, Li R, Yu Y, Kang F (2019) Surface-functionalized coating for lithium-rich cathode material to achieve ultra-high rate and excellent cycle performance. ACS Nano 13:11891–11900. https://doi.org/10.1021/acsnano.9b05960
Guo H, Wei Z, Jia K, Qiu B, Yin C, Meng F, Zhang Q, Gu L, Han S, Liu Y, Zhao H, Jiang W, Cui H, Xia Y, Liu Z (2019) Abundant nanoscale defects to eliminate voltage decay in Li-rich cathode materials. Energy Storage Mater 16:220–227. https://doi.org/10.1016/j.ensm.2018.05.022
Liu S, Liu Z, Shen X, Li W, Gao Y, Banis MN, Li M, Chen K, Zhu L, Yu R, Wang Z, Sun X, Lu G, Kong Q, Bai X, Chen L (2018) Surface doping to enhance structural integrity and performance of Li-rich layered oxide. Adv Energy Mater 8:1802105. https://doi.org/10.1002/aenm.201802105
Yin W, Grimaud A, Rousse G, Abakumov A M, Senyshyn A, Zhang L, Trabesinger S, Iadecola A, Foix D, Giaume D, Tarascon J-M (2020) Structural evolution at the oxidative and reductive limits in the first electrochemical cycle of Li1.2Ni0.13Mn0.54Co0.13O2. Nat Commun 11:1252. https://doi.org/10.1038/s41467-020-14927-4
Lu C, Yang S, Wu H, Zhang Y, Yang X, Liang T (2016) Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium doping. Electrochim Acta 209:448–455. https://doi.org/10.1016/j.electacta.2016.05.119
Wang R, He X, He L, Wang F, Xiao R, Gu L, Li H, Chen L (2013) Atomic structure of Li2MnO3 after partial delithiation and re-lithiation. Adv Energy Mater 3:1358–1367. https://doi.org/10.1002/aenm.201200842
Hong J, Seo DH, Kim SW, Gwon H, Oh ST, Kang K (2010) Structural evolution of layered Li1.2Ni0.2Mn0.6O2 upon electrochemical cycling in a Li rechargeable battery. J Mater Chem 20:10179. https://doi.org/10.1039/C0JM01971B
Yan P, Nie A, Zheng J, Zhou Y, Lu D, Zhang X, Xu R, Belharouak I, Zu X, Xiao J, Amine K, Liu J, Gao F, Shahbazian-Yassar R, Zhang JG, Wang C-M (215) Evolution of lattice structure and chemical composition of the surface reconstruction layer in Li1.2Ni0.2Mn0.6O2 cathode material for lithium ion batteries. Nano Lett 15:514–522. https://doi.org/10.1021/nl5038598
Lu C, Wu H, Zhang Y, Liu H, Chen B, Wu N, Wang S (2014) Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J Power Sources 267:682–691. https://doi.org/10.1016/j.jpowsour.2014.05.122
Xu B, Fell CR, Chi M, Meng YS (2011) Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: a joint experimental and theoretical study. Energy Environ Sci 4:2223–2233. https://doi.org/10.1039/C1EE01131F
Haruna AB, Ozoemena KI (2019) Effects of microwave irradiation on the electrochemical performance of manganese-based cathode materials for lithium-ion batteries. Curr Opin Electrochem 18:16–23. https://doi.org/10.1016/j.coelec.2019.08.005
Patrick L, Heino S, Martin SD, Petr N (2013) Oxygen release from high-energy xLi2MnO3·(1–x)LiMO2 (M = Mn, Ni, Co): electrochemical, differential electrochemical mass spectrometric, in situ pressure, and in situ temperature characterization. Electrochim Acta 93:114–119. https://doi.org/10.1016/j.electacta.2013.01.105
Zhou L, Yin Z, Ding Z, Li X, Wang Z, Wang Y (2018) Bulk and surface reconstructed Li-rich Mn-based cathode material for lithium ion batteries with eliminating irreversible capacity loss. J Electroanal Chem 829:7–15. https://doi.org/10.1016/j.jelechem.2018.09.043
Nayak PK, Grinblat J, Levi E, Markovsky B, Aurbach D (2016) Effect of cycling conditions on the electrochemical performance of high capacity Li and Mn-rich cathodes for Li-ion batteries. J Power Sources 318:9–17. https://doi.org/10.1016/j.jpowsour.2016.03.107
Liu Z, Zhang Z, Liu Y, Li L, Fu S (2019) Facile and scalable fabrication of K+-doped Li1.2Ni0.2Co0.08Mn0.52O2 cathode with ultra-high capacity and enhanced cycling stability for lithium ion batteries. Solid State Ionics 332:47–54. https://doi.org/10.1016/j.ssi.2018.12.021
Xiang Y, Li J, Wu X, Liu Z, Xiong L, He Z, Yin Z (2016) Synthesis and electrochemical characterization of Mg-doped Li-rich Mn-based cathode material. Ceram Int 42:8833–8838. https://doi.org/10.1016/j.ceramint.2016.02.128
Wang CC, Lin YC, Chou PH (2015) Mitigation of layer to spinel conversion of lithium-rich layer oxide cathode by substitution of Al for lithium ion battery. RSC Adv 5:68919–68928. https://doi.org/10.1039/C5RA11665A
Lengyel M, Shen KY, Lanigan DM, Martin JM, Zhang X, Axelbaum RL (2016) Trace level doping of lithium-rich cathode materials. J Mater Chem A 4:3538–3545. https://doi.org/10.1039/C5TA07764H
Song JH, Kapylou A, Choi HS, Yu BY, Matulevich E, Kang SH (2016) Suppression of irreversible capacity loss in Li-rich layered oxide by fluorine doping. J Power Sources 313:65–72. https://doi.org/10.1016/j.jpowsour.2016.02.058
Huang C, Wang Z, Wang H, Huang D, Zhao J-W, Zhao S-X (2022) In-situ construction of extra ion-store sites and fast ion-diffusion channels for lithium-rich manganese-based oxides cathode. J Power Sources 535:231437. https://doi.org/10.1016/j.jpowsour.2022.231437
Zhou L, Yin Z, Tian H, Ding Z, Li X, Wang Z, Guo H (2018) Spinel-embedded and Li3PO4 modified Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for High-Performance Li-Ion batteries. Appl Surf Sci 456:763–770. https://doi.org/10.1016/j.apsusc.2018.06.114
Deng B, Chen Y, Wu P, Han J, Li Y, Zheng H, Xie Q, Wang L, Peng DL (2019) Lithium-rich layered oxide nanowires bearing porous structures and spinel domains as cathode materials for lithium-ion batteries. J Power Sources 418:122–129. https://doi.org/10.1016/j.jpowsour.2019.02.036
Dai D, Yan D, Li B, Chang K, Chang Z, Tang H, Li Y, Zhou S (2017) A facile and scalable self-assembly strategy to prepare two-dimensional nanoplates: a precursor for a Li-rich layered cathode material Li1.2Mn0.54Ni0.13Co0.13O2 with high capacity and rate Performance. Electrochim Acta 235:632–639. https://doi.org/10.1016/j.electacta.2017.03.148
Zhu X, Tang J, Huang H, Lin T, Luo B, Wang L (2020) Hollow structured cathode materials for rechargeable batteries. Sci Bull 65:496–512. https://doi.org/10.1016/j.scib.2019.12.008
Yuan X, Xu Qj, Wang C, Liu X, Liu H, Xia Y (2015) A facile and novel organic coprecipitation strategy to prepare layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with high capacity and excellent cycling stability. J Power Sources 279:157–164. https://doi.org/10.1016/j.jpowsour.2014.12.148
Zhang Y, Song Q, Wang T, Zhao Q, Zhang R, Zhao J (2019) Crystal preferred orientation of Li2MnO3·LiMO2 (M=Mn Co, Ni) nano-particals: Relevance to electrochemical behavior for lithium battery cathode materials. J Power Sources 413:425–431. https://doi.org/10.1016/j.jpowsour.2018.12.069
Ma Q, Li R, Zheng R, Liu Y, Huo H, Dai C (2016) Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes via selenium doping to stabilize oxygen. J Power Sources 331:112–121. https://doi.org/10.1016/j.jpowsour.2016.08.137
Yu R, Wang G, Liu M, Zhang X, Wang X, Shu H, Yang X, Huang W (2016) Mitigating voltage and capacity fading of lithium-rich layered cathodes by lanthanum doping. J Power Sources 335:65–75. https://doi.org/10.1016/j.jpowsour.2016.10.042
Song B, Lai MO, Lu L (2012) Influence of Ru substitution on Li-rich 0.55Li2MnO3·0.45LiNi1/3Co1/3Mn1/3O2 cathode for Li-ion batteries. Electrochim Acta 80:187–195. https://doi.org/10.1016/j.electacta.2012.06.118
Nayak PK, Grinblat J, Levi E, Levi M, Markovsky B, Aurbach D (2017) Understanding the influence of Mg doping for the stabilization of capacity and higher discharge voltage of Li- and Mn-rich cathodes for Li-ion batteries. Phys Chem Chem Phys 19:6142–6152. https://doi.org/10.1039/C6CP07383B
Sorboni YG, Arabi H, Kompany A (2019) Effect of Cu doping on the structural and electrochemical properties of lithium-rich Li1.2Mn0.50Ni0.125Co0.125O2 nanopowders as a cathode material. Ceram Int 45:2139–2145. https://doi.org/10.1016/j.ceramint.2018.10.122
Zhao J, Wang Z, Guo H, Li X, He Z, Li T (2015) Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. Ceram Int 41:11396–11401. https://doi.org/10.1016/j.ceramint.2015.05.102
Wang CC, Lin YC, Chiu KF (2017) Alleviation of voltage fade of lithium-rich layered oxide cathodes of Li-ion battery by incorporation of Cr. J Alloy Compd 721:600–608. https://doi.org/10.1016/j.jallcom.2017.06.024
Li H, Jian Z, Yang P, Li J, Xing Y, Zhang S (2020) Niobium doping of Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials with enhanced structural stability and electrochemical performance. Ceram Int 46:23773–23779. https://doi.org/10.1016/j.ceramint.2020.06.152
Liu X, Yu B, Wang M, Jin Y, Fu Z, Chen J, Ma Z, Guo B, Huang Y, Li X (2022) Electrochemical performances of niobium-doped new spherical low-cobalt Li-rich Mn-based Li1.14Mn0.476Ni0.254Co0.048Al0.016O2 cathode. Mater Today Commun 32:104170. https://doi.org/10.1016/j.mtcomm.2022.104170
Chen J, Zhao N, Ban KJ, Wang YN, Zhang XY (2019) Superior electrochemical properties of Li[Li0.2Ni0.18Mn0.6Mg0.02]O2 cathode material with hierarchical micronanostructure for lithium ion batteries. J Alloy Compd 805:673–679. https://doi.org/10.1016/j.jallcom.2019.07.136
Yu T, Li J, Xu G, Li J, Ding F, Kang F (2017) Improved cycle performance of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 by Ga doping for lithium ion battery cathode material. Solid State Ionics 301:64–71. https://doi.org/10.1016/j.ssi.2017.01.008
Du J, Shan Z, Zhu K, Liu X, Tian J, Du H (2015) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by doping with molybdenum for Lithium battery. J Solid State Electrochem 19:1037–1044. https://doi.org/10.1007/s10008-014-2706-6
Guo H, Xia Y, Zhao H, Yin C, Jia K, Zhao F, Liu Z (2017) Stabilization effects of Al doping for enhanced cycling performances of Li-rich layered oxides. Ceram Int 43:13845–13852. https://doi.org/10.1016/j.ceramint.2017.07.107
Tang T, Zhang HL (2016) Synthesis and electrochemical performance of lithium-rich cathode material Li[Li0.2Ni0.15Mn0.55Co0.1-xAlx]O2. Electrochim Acta 191:263–269. https://doi.org/10.1016/j.electacta.2016.01.066
Yi TF, Li YM, Cai XD, Yang SY, Zhu YR (2017) Fe-stabilized Li-rich layered Li1.2Mn0.56Ni0.16Co0.08O2 oxide as a high performance cathode for advanced lithium-ion batteries. Mater Today Energy 4:25–33. https://doi.org/10.1016/j.mtener.2017.03.005
Kang S, Qin H, Fang Y, Li X, Wang Y (2014) Preparation and electrochemical performance of yttrium-doped Li[Li0.20Mn0.534Ni0.133Co0.133]O2 as cathode material for lithium-ion batteries. Electrochim Acta 144:22–30. https://doi.org/10.1016/j.electacta.2014.06.155
Bao L, Yang Z, Chen L, Su Y, Lu Y, Li W, Yuan F, Dong J, Fang Y, Ji Z, Chen S, Wu F (2019) The effects of trace Yb doping on the electrochemical performance of layered Li-rich oxide. Chemsuschem 12:2294–2301. https://doi.org/10.1002/cssc.201900226
Nayak PK, Grinblat J, Levi M, Levi E, Kim S, Choi JW, Aurbach D (2016) Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv Energy Mater 6:1502398. https://doi.org/10.1002/aenm.201502398
He H, Guan L, Dong J, Chang C, Zhang D (2020) Improving the electrochemical behavior of Li1.27Cr0.2Mn0.53O2 cathode via the incorporating Cu2+ cations into the transition metal slab. Electrochim Acta 330:135313. https://doi.org/10.1016/j.electacta.2019.135313
Ramesha RN, Laisa CP, Ramesha K (2017) Improving electrochemical stability by transition metal cation doping for manganese in lithium-rich layered cathode, Li1.2Ni0.13Co0.13Mn0.54-xMxO2 (M = Co, Cr and Fe). Electrochim Acta 249:377–386. https://doi.org/10.1016/j.electacta.2017.08.039
Zang Y, Ding C, Wang X, Wen Z, Chen C (2015) Molybdenum-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with high specific capacity and improved rate performance. Electrochim Acta 168:234–239. https://doi.org/10.1016/j.electacta.2015.03.223
Zang Y, Sun X, Tang ZF, Xiang HF, Chen CH (2016) Vanadium-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with a high specific capacity and improved rate performance. RSC Adv 6:30194–30198. https://doi.org/10.1039/C6RA02472F
Ma Z, Huang J, Quan J, Mei L, Guo J, Li D (2016) Improved electrochemical performances of layered lithium rich oxide 0.6Li[Li1/3Mn2/3]O2·0.4LiMn5/12Ni5/12Co1/6O2 by Zr doping. RSC Adv 6:20522–20531. https://doi.org/10.1039/C5RA22330J
Qiao QQ, Qin L, Li GR, Wang YL, Gao XP (2015) Sn-stabilized Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as cathode for advanced lithium-ion batteries. J Mater Chem A 3:17627–17634. https://doi.org/10.1039/C5TA03415A
Xie K, Qian J, Zhou Y, Chen Z, Lin Y, Chen F, Shen Z (2018) Influences of Gd3+ doping modification on the crystal microstructure and electrochemical performance of Li1.20[Mn0.52Ni0.20Co0.08]O2 as cathode for Lithium-ion batteries. Powder Technol 339:838–845. https://doi.org/10.1016/j.powtec.2018.08.059
Li N, An R, Su Y, Wu F, Bao L, Chen L, Zheng Y, Shou H, Chen S (2013) The role of yttrium content in improving electrochemical performance of layered lithium-rich cathode materials for Li-ion batteries. J Mater Chem A 1:9760–9767. https://doi.org/10.1039/C3TA11665D
Li X, Xin H, Liu Y, Li D, Yuan X, Qin X (2015) Effect of niobium doping on the microstructure and electrochemical properties of lithium-rich layered Li[Li0.2Ni0.2Mn0.6]O2 as cathode materials for lithium ion batteries. RSC Adv 5:45351–45358. https://doi.org/10.1039/C5RA01798J
Zhao L, Wu Q, Wu J (2018) Improving rate performance of cathode material Li1.2Mn0.54Co0.13Ni0.13O2 via niobium doping. J Solid State Electrochem 22:2141–2148. https://doi.org/10.1007/s10008-018-3912-4
Chen J, Wang Y, Zhao N, Liu ZQ (2019) Hierarchical micro-nanostructured and Al3+-doped Li1.2Ni0.2Mn0.6O2 active materials with enhanced electrochemical properties as cathode materials for Li-ion batteries. Scripta Mater 171:47–51. https://doi.org/10.1016/j.scriptamat.2019.06.022
He A (2017) Effects of Al doping on structure and electrochemical performance of Li1.2Ni0.2Mn0.6O2. Inorg Chem Indust 49(7):74–77. http://d.wanfangdata.com.cn/periodical/wjygy201707019
Li H, Guo H, Wang Z, Wang J, Li X, Chen N, Gui W (2018) Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes by chromium doping. Int J Hydrogen Energy 43:11109–11119. https://doi.org/10.1016/j.ijhydene.2018.04.203
Liu X, Huang T, Yu A (2014) Fe doped Li1.2Mn0.6-x/2Ni0.2-x/2FexO2 (x ≤ 0.1) as cathode materials for lithium-ion batteries. Electrochim Acta 133:555–563. https://doi.org/10.1016/j.electacta.2014.04.085
Wu F, Kim GT, Kuenzel M, Zhang H, Asenbauer J, Geiger D, Kaiser U, Passerini S (2019) Elucidating the effect of iron doping on the electrochemical performance of cobalt-free lithium-rich layered cathode materials. Adv Energy Mater 9:1902445. https://doi.org/10.1002/aenm.201902445
Pan W, Peng W, Guo H, Wang J, Wang Z, Li H, Shih K (2017) Effect of molybdenum substitution on electrochemical performance of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 cathode material. Ceram Int 43:14836–14841. https://doi.org/10.1016/j.ceramint.2017.07.232
Yuan X, Xu Q, Liu X, Shen W, Liu H, Xia Y (2016) Excellent rate performance and high capacity of Mo doped layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 derived from an improved coprecipitation approach. Electrochim Acta 207:120–129. https://doi.org/10.1016/j.electacta.2016.04.180
Han D, Park K, Park J, Yun D, Son Y (2018) Selective doping of Li-rich layered oxide cathode materials for high-stability rechargeable Li-ion batteries. J Ind Eng Chem 68:180–186. https://doi.org/10.1016/j.jiec.2018.07.044
Chen H, Hu Q, Huang Z, He Z, Wang Z, Guo H, Li X (2016) Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram Int 42:263–269. https://doi.org/10.1016/j.ceramint.2015.08.104
Dong S, Zhou Y, Hai C, Zeng J, Sun Y, Shen Y, Li X, Ren X, Sun C, Zhang G, Wu Z (2020) Understanding electrochemical performance improvement with Nb doping in lithium-rich manganese-based cathode materials. J Power Sources 462:228185. https://doi.org/10.1016/j.jpowsour.2020.228185
Hu X, Guo H, Peng W, Wang Z, Li X, Hu Q (2018) Effects of Nb doping on the performance of 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion batteries. J Electroanal Chem 822:57–65. https://doi.org/10.1016/j.jelechem.2018.05.015
Uzun D (2015) Boron-doped Li1.2Mn0.6Ni0.2O2 as a cathode active material for lithium ion battery. Solid State Ion 281:73–81. https://doi.org/10.1016/j.ssi.2015.09.008
Pan L, Xia Y, Qiu B, Zhao H, Guo H, Jia K, Gu Q, Liu Z (2016) Structure and electrochemistry of B doped Li(Li0.2Ni0.13Co0.13Mn0.54)1-xBxO2 as cathode materials for lithium-ion batteries. J Power Sources 327:273–280. https://doi.org/10.1016/j.jpowsour.2016.07.064
Zhao Y, Xia M, Hu X, Zhao Z, Wang Y, Lv Z (2015) Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim Acta 174:1167–1174. https://doi.org/10.1016/j.electacta.2015.05.068
Wu Z, Wang D, Gao Z, Yue H, Liu W (2015) Effect of Cu substitution on structures and electrochemical properties of Li [NiCo1−xCuxMn]1/3O2 as cathode materials for lithium ion batteries. Dalton Trans 44:18624–18631. https://doi.org/10.1039/C5DT02552D
Zhang K, Li B, Zuo Y, Song J, Shang H, Ning F, Xia D (2019) Voltage decay in layered Li-rich Mn-based cathode materials. Electrochem Energy Rev 2:606–623. https://doi.org/10.1007/s41918-019-00049-z
Rozier P, Tarascon JM (2015) Review—Li-rich layered oxide cathodes for next-generation li-ion batteries: chances and challenges. J Electrochem Soc 162:A2490–A2499. https://doi.org/10.1149/2.0111514jes
Liu C, Wu M, Hang S, Xu T, Yang G, Ji H, Yang Y (2019) Synthesis of Li1.147K0.026Mn0.582Ni0.25O2 cathode material with high rate cyclic performance and the application to lithium-ion full cells. J Alloy Compd 787:700–710. https://doi.org/10.1016/j.jallcom.2019.02.151
Wang D, Liu M, Wang X, Yu R, Wang G, Ren Q, Yang X (2016) Facile synthesis and performance of Na-doped porous lithium-rich cathodes for lithium ion batteries. RSC Adv 6:57310–57319. https://doi.org/10.1039/C6RA09042G
Liu Y, Liu D, Wu H-H, Fan X, Dou A, Zhang Q, Su M (2018) Improved cycling stability of Na-doped cathode materials Li1.2Ni0.2Mn0.6O2 via a facile synthesis. ACS Sustain Chem Eng 6:13045–13055. https://doi.org/10.1021/acssuschemeng.8b02552
Li N, He YS, Wang X, Zhang W, Ma ZF, Zhang D (2017) Incorporation of rubidium cations into Li1.2Mn0.54Co0.13Ni0.13O2 layered oxide cathodes for improved cycling stability. Electrochim Acta 231:363–370. https://doi.org/10.1016/j.electacta.2017.01.137
Ding X, Li Y, Deng M, Wang S, Aqsa Y, Hu Q, Chen C (2019) Cesium doping to improve the electrochemical performance of layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material. J Alloy Compds 791:100–108. https://doi.org/10.1016/j.jallcom.2019.03.297
Choi A, Lim J, Kim H, Jung SC, Lim HW, Kim H, Kwon M, Han YK, Oh SM, Lee KT (2018) Site-selective in situ electrochemical doping for Mn-rich layered oxide cathode materials in lithium-ion batteries. Adv Energy Mater 8:1702514. https://doi.org/10.1002/aenm.201702514
Huang C, Wang Z, Wang H, Huang D, He Y-B, Zhao S-X (in press) Mg2+ doping into Li sites to improve anionic redox reversibility and thermal stability of lithium-rich manganese-based oxides cathode. Mater Today Energy. https://doi.org/10.1016/j.mtener.2022.101116
Yan W, Xie Y, Jiang J, Sun D, Ma X, Lan Z, Jin Y (2018) Enhanced rate performance of Al-doped Li-rich layered cathode material via nucleation and post-solvothermal method. ACS Sustainable Chem Eng 6:4625–4632. https://doi.org/10.1021/acssuschemeng.7b03634
Sallard S, Billaud J, Sheptyakov D, Novák P, Villevieille C (2020) Cr-doped Li-rich nickel cobalt manganese oxide as positive electrode material in Li-ion batteries to enhance cycling stability. ACS Appl Energy Mater 3:8646–8657. https://doi.org/10.1021/acsaem.0c01235
He T, Chen L, Su Y, Lu Y, Bao L, Chen G, Zhang Q, Chen S, Wu F (2019) The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J Power Sources 441:227195. https://doi.org/10.1016/j.jpowsour.2019.227195
Song C, Feng W, Wang X, Shi Z (2020) Improved storage capability and cycle stability in a Li-riched cathode by substituted Al. J Electroanal Chem 862:113962. https://doi.org/10.1016/j.jelechem.2020.113962
Korobov DD, Mitrofanov IV, Pushnitsa KA, Kim AE, Koshtyal YM, Pechen LS, Popovich AA, Maximov MY (2020) Features of improved capacity at high discharge rate of K-doped Li-rich cathodes for LIBs. Mater Today 30:778–783. https://doi.org/10.1016/j.matpr.2020.01.566
Yang M, Hu B, Geng F, Li C, Lou X, Hu B (2018) Mitigating voltage decay in high-capacity Li1.2Ni0.2Mn0.6O2 cathode material by surface K+ doping. Electrochim Acta 291:278–286. https://doi.org/10.1016/j.electacta.2018.09.134
Liu C, Wu M, Guo Z, Luo X, Ji H, Yang G, Hou W (2020) Preparation and characterization of Li1.167-xKxMn0.583Ni0.25O2 (x=0, 0.025, 0.05 and 0.075) as cathode materials for highly reversible lithium-ion batteries. Electrochim Acta 341:136014. https://doi.org/10.1016/j.electacta.2020.136014
Zhang P, Zhai X, Huang H, Zhou J, Li X, He Y, Guo Z (2020) Suppression of structural phase transformation of Li-rich Mn-based layered cathode materials with Na ion substitution strategy. Electrochim Acta 349:136402. https://doi.org/10.1016/j.electacta.2020.136402
Lim SN, Seo JY, Jung DS, Ahn W, Song HS, Yeon SH, Park SB (2015) Rate capability for Na-doped Li1.167Ni0.18Mn0.548Co0.105O2 cathode material and characterization of Li-ion diffusion using galvanostatic intermittent titration technique. J Alloy Compd 623:55–61. https://doi.org/10.1016/j.jallcom.2014.09.203
Zhou Y, Shan W, Hou X, Lam K, Zhao X, Liu X, Wu Y (2020) Study of spherical Li1.2-xNaxMn0.534Ni0.133Co0.133O2 cathode based on dual Li+/Na+ transport system for Li-ion batteries. Solid State Ion 350:115326. https://doi.org/10.1016/j.ssi.2020.115326
Yu R, Wang X, Fu Y, Wang L, Cai S, Liu M, Lu B, Wang G, Wang D, Ren Q, Yang X (2016) Effect of magnesium doping on properties of lithium-rich layered oxide cathodes based on a one-step co-precipitation strategy. J Mater Chem A 4:4941–4951. https://doi.org/10.1039/C6TA00370B
Lou M, Fan S, Yu HT, Xie Y, Zhang Q, Zhu YR, Yi TF, Tian GH (2018) Mg-doped Li1.2Mn0.54Ni0.13Co0.13O2 nano flakes with improved electrochemical performance for lithium-ion battery application. J Alloy Compd 739:607–615. https://doi.org/10.1016/j.jallcom.2017.12.286
Wang Y, Shang K, He W, Ai X, Cao Y, Yang H (2015) Magnesium-doped Li1.2[Co0.13Ni0.13Mn0.54]O2 for lithium-ion battery cathode with enhanced cycling stability and rate capability. ACS Appl Mater Inter 7:13014–13021. https://doi.org/10.1021/acsami.5b03125
Jin Y, Xu Y, Ren F, Ren P (2019) Mg-doped Li1.133Ni0.2Co0.2Mn0.467O2 in Li site as high-performance cathode material for Li-ion batteries. Solid State Ion 336:87–94. https://doi.org/10.1016/j.ssi.2019.03.020
Yan W, Xie Y, Jiang J, Sun D, Ma X, Lan Z (2018) Enhanced rate performance of Al-doped Li-rich layered cathode material via nucleation and post-solvothermal method. ACS Sustain Chem Eng 6:4625–4632. https://doi.org/10.1021/acssuschemeng.7b03634
Kitchaev DA, Lun Z, Richards WD, Ji H, Clement RJ, Balasubramanian M, Kwon DH, Dai K, Papp JK, Lei T, McCloskey BD, Yang W, Lee J, Ceder G (2018) Design principles for high transition metal capacity in disordered rocksalt li-ion cathodes. Energy Environ Sci 11:2159–2171. https://doi.org/10.1039/C8EE00816G
Kang SH, Amine K (2005) Layered Li(Li0.2Ni0.15+0.5zCo0.10Mn0.55–0.5z)O2 - ZFz cathode materials for Li-ion secondary batteries. J Power Sources 146:654−657. https://doi.org/10.1016/j.jpowsour.2005.03.152
Li L, Song BH, Chang YL, Xia H, Yang JR, Lee KS, Lu L (2015) Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J. Power Sources 283:162–170. https://doi.org/10.1016/j.jpowsour.2015.02.085
Xie D, Zhou W, Lin K, Hu C, Zheng P, Hou X, Lam K (2019) Doping effect of flfluoride anion on microstructural and electrochemical properties of lithium-rich cathode materials. Mater Lett 253:82–85. https://doi.org/10.1016/j.matlet.2019.06.047
Pang WK, Lin HF, Peterson VK, Lu CZ, Liu CE, Liao SC, Chen JM (2017) Effects of fluorine and chromium doping on the performance of lithium-rich Li1+xMO2 (M = Ni, Mn, Co) positive electrodes. Chem Mater 29:10299–10311. https://doi.org/10.1021/acs.chemmater.7b02930
An J, Shi L, Chen G, Li M, Liu H, Yuan S, Chen S, Zhang D (2017) Insights into the stable layered structure of a Li-rich cathode material for lithium-ion batteries. J Mater Chem A 5:19738–19744. https://doi.org/10.1039/C7TA05971J
Watanabe A, Yamamoto K, Uchiyama T, Matsunaga T, Hayashi A, Maeda K, Kageyama H, Uchimoto Y (2020) Capacity improvement by nitrogen doping to lithium-rich cathode materials with stabilization effect of oxide ions redox. ACS Appl Energy Mater 3:4162–4167. https://doi.org/10.1021/acsaem.0c00564
Vanaphuti P, Bong S, Ma L, Ehrlich S, Wang Y (2020) Systematic study of different anion doping on the electrochemical performance of cobalt-free lithium-manganese-rich layered cathode. ACS Appl Energy Mater 3:4852–4859. https://doi.org/10.1021/acsaem.0c00439
Zhang HZ, Qiao QQ, Li GR, Gao XP (2014) PO43- polyanion-doping for stabilizing Li-rich layered oxides as cathode materials for advanced lithium-ion batteries. J Mater Chem A 2:7454–7460. https://doi.org/10.1039/C4TA00699B
Vanaphuti P, Chen J, Cao J, Bigham K, Chen B, Yang L, Chen H, Wang Y (2019) Enhanced electrochemical performance of the lithium-manganese-rich cathode for Li-ion batteries with Na and F Co-doping. ACS Appl Mater Inter 11:37842–37849. https://doi.org/10.1021/acsami.9b13838
Liu D, Fan X, Li Z, Liu T, Sun M, Qian C, Ling M, Liu Y, Liang C (2019) A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries. Nano Energy 58:786–796. https://doi.org/10.1016/j.nanoen.2019.01.080
Zhang P, Zhai X, Huang H, Zhou J, Li X, He Y, Guo Z (2020) Synergistic Na+ and F− co-doping modification strategy to improve the electrochemical performance of Li-rich Li1·20Mn0·54Ni0·13Co0·13O2 cathode. Ceram Int 46:24723–24736. https://doi.org/10.1016/j.ceramint.2020.06.263
Vanaphuti P, Wang F, Bai J, Ma L, Ehrlich S, Kisslinger K, Wang Y (2020) Unraveling Na and F coupling effects in stabilizing Li, Mn-rich layered oxide cathodes via local ordering modification. Energy Storage Mater 31:459–469. https://doi.org/10.1016/j.ensm.2020.08.003
Lim SN, Seo JY, Jung DS, Park SB, Yeon SH (2015) The crystal structure and electrochemical performance of Li1.167Mn0.548Ni0.18Co0.105O2 composite cathodes doped and co-doped with Mg and F. J Electroanal Chem 740:88–94. https://doi.org/10.1016/j.jelechem.2015.01.010
Guo B, Zhao J, Fan X, Zhang W, Li S, Yang Z, Chen Z, Zhang W (2017) Aluminum and fluorine co-doping for promotion of stability and safety of lithium-rich layered cathode material. Electrochim Acta 236:171–179. https://doi.org/10.1016/j.electacta.2017.03.133
Gu H, Liu T, Liu T, Zhou Y, Xu M, Chen F (2019) Improving the electrochemical properties of Mn-rich Li1.20[Mn0.54Ni0.13Co0.13]O2 by Nb and F co-doping. Solid State Ion 336:129–138. https://doi.org/10.1016/j.ssi.2019.03.018
Chen G, An J, Meng Y, Yuan C, Matthews B, Dou F, Shi L, Zhou Y, Song P, Wu G, Zhang D (2019) Cation and anion Co-doping synergy to improve structural stability of Li- and Mn-rich layered cathode materials for lithium-ion batteries. Nano Energy 57:157–165. https://doi.org/10.1016/j.nanoen.2018.12.049
Liu Y, Ning D, Zheng L, Zhang Q, Gu L, Gao R, Zhang J, Fran A, Schumacher G, Liu X (2018) Improving the electrochemical performances of Li-rich Li1.20Ni0.13Co0.13Mn0.54O2 through a cooperative doping of Na+ and PO43- with Na3PO4. J Power Sources 375:1–10. https://doi.org/10.1016/j.jpowsour.2017.11.042
Liu Y, He B, Li Q, Liu H, Qiu L, Liu J, Xiang W, Liu Y, Wang G, Wu Z, Guo X (2020) Relieving capacity decay and voltage fading of Li1.2Ni0.13Co0.13Mn0.54O2 by Mg2+ and PO43- dual doping. Mater Res Bull 130:110923. https://doi.org/10.1016/j.materresbull.2020.110923
Yu Y, Yang Z, Zhong J, Liu Y, Li J, Wang X, Kang F (2020) A Simple dual-ion doping method for stabilizing Li-rich materials and suppressing voltage decay. ACS Appl Mater Inter 12:13996–14004. https://doi.org/10.1021/acsami.0c00944
Li C, Cai X, Fu X, Zhang N, Ding H, Wang P, Zhou X, Song L, Huang J, Li S (2022) Cation and polyanion co-doping synergy to improve electrochemical performances of Li-rich manganese-based cathode materials. J Alloys Compd 924:166527. https://doi.org/10.1016/j.jallcom.2022.166527
Li B, Wang X, Gao Y, Wang B, Qiu J, Cheng X, Dai D (2019) Improving rate performances of Li-rich layered oxide by the co-doping of Sn and K ions. J Materiomics 5:149–155. https://doi.org/10.1016/j.jmat.2019.01.005
Jiang W, Zhang C, Feng Y, Wei B, Chen L, Zhang R, Ivey DG, Wang P, Wei W (2020) Achieving high structure and voltage stability in cobalt-free Li-rich layered oxide cathodes via selective dual-cation doping. Energy Storage Mater 32:37–45. https://doi.org/10.1016/j.ensm.2020.07.035
Sun Y, Zhang L, Dong S, Zeng J, Shen Y, Li X, Ren X, Ma L, Hai C, Zhou Y (2022) Improving the electrochemical performances of Li-rich Li1.2Ni0.13Co0.13Mn0.54O2 through cooperative doping of Na+ and Mg2+. Electrochimica Acta 414:140169. https://doi.org/10.1016/j.electacta.2022.140169
Sun Y, Wu Q, Zhao L (2019) A new doping element to improve the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 materials for Li-ion batteries. Ceram Int 45:1339–1347. https://doi.org/10.1016/j.ceramint.2018.06.154
Peng Z, Mu K, Cao Y, Xu L, Du K, Hu G (2019) Enhanced electrochemical performance of layered Li-rich cathode materials for lithium ion batteries via aluminum and boron dual-doping. Ceram Int 45:4184–4192. https://doi.org/10.1016/j.ceramint.2018.11.087
Xie D, Li G, Li Q, Fu C, Fan J, Li L (2016) Improved cycling stability of cobalt-free Li-rich oxides with a stable interface by dual doping. Electrochim Acta 196:505–516. https://doi.org/10.1016/j.electacta.2016.02.210
Liang Y, Li S, Xie J, Yang L, Li W, Li C, Ai L, Fu X, Cui X, Shangguan X (2019) Synthesis and electrochemical characterization of Mg-Al co-doped Li-rich Mn-based cathode materials. New J Chem 43:12004–12012. https://doi.org/10.1039/C9NJ01539F
Liu Y, Li R, Li J, Yang Z, Zhong J, Wang Z, Kang F (2019) A high-performance Ce and Sn co-doped cathode material with enhanced cycle performance and suppressed voltage decay for lithium ion batteries. Ceram Int 45:20780–20787. https://doi.org/10.1016/j.ceramint.2019.07.064
Ghorbanzadeh M, Allahyari E, Riahifar R, Hadavi SMM (2018) Effect of Al and Zr co-doping on electrochemical performance of cathode Li[Li0.2Ni0.13Co0.13Mn0.54]O2 for Li-ion battery. J Solid State Electrochem 22:1155–1163. https://doi.org/10.1007/s10008-017-3824-8
Celeste A, Girardi F, Gigli L, Pellegrini V, Silvestri L, Brutti S (2022) Impact of Overlithiation and Al doping on the battery performance of Li-rich layered oxide materials. Electrochim Acta 428:140737. https://doi.org/10.1016/j.electacta.2022.140737
Lee Y, Kim TH, Kwon YK, Shin J, Cho EA (2020) Selective formation of the Li4Mn5O12 surface spinel phase in sulfur-doped Li-excess-layered cathode materials for improved cycle life. ACS Sustain Chem Eng 8:8037–8048. https://doi.org/10.1021/acssuschemeng.0c02687
Jia X, Wei L, Xu L, Hu Y, Guo H, Li Y (2020) Nb5þ doped Li1.20Mn0.54Ni0.13Co0.13O2 with Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) surface modification as advanced cathode material for Li-ion batteries. J Alloy Compd 832:154986. https://doi.org/10.1016/j.jallcom.2020.154986
Li S, Fu X, Liang Y, Wang S, Zhou X, Dong H, Tuo K, Gao C, Cui X-L (2020) Enhanced structural stability of boron-doping layered @ spinel @ carbon heterostructured lithium-rich manganese-based cathode materials. ACS Sustain Chem Eng 8:9311–9324. https://doi.org/10.1021/acssuschemeng.0c00870
Mei J, Chen Y, Xu W, He W, Wang L, Xie Q, Peng D-L (2022) Multi-strategy synergistic Li-rich layered oxides with fluorine-doping and surface coating of oxygen vacancy bearing CeO2 to achieve excellent cycling stability. Chem Eng J 431:133799
He W, Liu P, Zhou Y, Zheng H, Zheng Z, Liu B, Yuan J, Zhang Q, Wang L, Luo Q, Xie Q, Qu B, Peng D-L (2020) A novel morphology-controlled synthesis of Na+-doped Li- and Mn-rich cathodes by the self-assembly of amphiphilic spherical micelles. Sustain Mater Techno 25:e00171. https://doi.org/10.1016/j.susmat.2020.e00171
Xu C, Li J, Sun Jie, Zhang W, Ji B (2022) Li-rich layered oxide single crystal with Na doping as a high-performance cathode for Li ion batteries. J Alloys Compd 895:162613. https://doi.org/10.1016/j.jallcom.2021.162613
Ouyang CY, Shi SQ, Wang ZX, Li H, Huang XJ, Chen LQ (2004) The effect of Cr doping on Li ion diffusion in LiFePO4 from first principles investigations and Monte Carlo simulations. J Phys: Condens Matter 16:2265–2272. https://doi.org/10.1088/0953-8984/16/13/007
Wang L, Maxisch T, Ceder G (2007) A First-principles approach to studying the thermal stability of oxide cathode materials. Chem Mater 19:543–552. https://doi.org/10.1021/cm0620943
Kong F, Longo RC, Park M-S, Yoon J, Yeon D-H, Park J-H, Wang W-H, Santosh KC, Doo S-G, Cho K (2015) Ab initio study of doping effects on LiMnO2 and Li2MnO3 cathode materials for Li-ion batteries. J Mater Chem A 3:8489–8500. https://doi.org/10.1039/C5TA01445J
Kong F, Liang C, Longo RC, Yeon D-H, Zheng Y, Park J-H, Doo S-G, Cho K (2016) Conflicting roles of anion doping on the electrochemical performance of Li-ion battery cathode materials. Chem Mater 28:6942–6952. https://doi.org/10.1021/acs.chemmater.6b02627
Lim J-M, Hwang T, Park M-S, Cho M, Cho K (2016) Design of a p-type electrode for enhancing electronic conduction in high-Mn, Li-rich oxides. Chem Mater 28:8201–8209. https://doi.org/10.1021/acs.chemmater.6b03032
Kim D, Lim J-M, Park M-S, Cho K, Cho M (2016) Phase separation and d electronic orbitals on cyclic degradation in Li–Mn–O compounds: first-principles multiscale modeling and experimental observations. ACS Appl Mater Inter 8:16631–16639. https://doi.org/10.1021/acsami.6b01595
Chen C, Liang Q, Chen Z, Zhu W, Wang Z, Li Y, Wu X, Xiong X (2021) Phenoxy radical-induced formation of dual-layered protection film for high-rate and dendrite-free lithium-metal anodes. Angew Chem Int Ed 60:26718–26724. https://doi.org/10.1002/anie.202110441
Liu D, Xiong X, Liang Q, Wu X, Fu H (2021) An inorganic-rich SEI induced by LiNO3 additive for a stable lithium metal anode in carbonate electrolyte. Chem Commun 57:9232–9235. https://doi.org/10.1039/D1CC03676A
Okubo M, Yamada A (2017) molecular orbital principles of oxygen-redox battery electrodes. ACS Appl Mater Interfaces 9:36463–36472. https://doi.org/10.1021/acsami.7b09835
Seo DH, Lee J, Urban A, Malik R, Kang SY, Ceder G (2016) The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat Chem 8:692−697. https://www.nature.com/articles/nchem.2524
Chen J, Chen H, Deng W, Gao X, Yin S, Mei Y, Zhang S, Ni L, Gao J, Liu H, Tian Y, Yang L, Deng X, Zou G, Hou H, Xie J, Ji X (2022) p-type orbital hybridization and reactive oxygen quenching induced by Se-doping for Li-rich Mn-based oxide cathode. Energy Storage Mater 51:671–682. https://doi.org/10.1016/j.ensm.2022.06.004
Funding
The author would like to thank the science and technology plan projects of Baoji (16RKX1-18), the Phytochemistry Key Laboratory of Shaanxi Province (grant no. 18JS007), and the Natural Science Foundation of China (51702006) for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shumei, D., Dan, T., Ping, L. et al. Research progress and prospect in element doping of lithium-rich layered oxides as cathode materials for lithium-ion batteries. J Solid State Electrochem 27, 1–23 (2023). https://doi.org/10.1007/s10008-022-05294-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05294-9