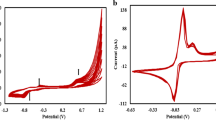
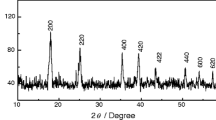
Abstract
In this work, we investigate the properties of manganese dioxide (MnO2) and graphene oxide (GO) to develop a new photoelectrochemical (PEC) sensor-based MnO2-GO heterojunction on stainless steel electrode for chemical detection of copper (Cu(II)) cation. During the PEC detection process and using chronoamperometry, the photocurrent density increases gradually with the addition of incremental Cu2+ concentration, achieving a relatively low detection limit (0.9120 µM) and a linear interval range from 0.01 to 110 µM with high selectivity and stability under neutral aqueous solution (pH 7.00). In addition, the PEC change of electrode state in presence of Cu(II) cation requires the oxidized state of MnO2-GO heterojunction; this can be reached by applying anodic potential to the electrode (E = + 0.8 V). Thus, a switchable, low-cost, regenerative, and sensitive PEC sensor based on the change of MnO2-GO heterojunction surface state permits the selective detection of copper.
Graphical abstract



source: 500-W xenon lamp with AM 1.5 G filter; the light intensity was adjusted to 100 mW cm–2

source: 500-W xenon lamp with AM 1.5 G filter; the light intensity was adjusted to 100 mW cm–2
Similar content being viewed by others
References
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/waste water by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Sharma Y, Srivastava V, Singh V, Kaul S, Weng C (2009) Nanoadsorbents for the removal of metallic pollutants from water and wastewater. Environ Technol 30(6):583–609
Wu M, Liu C, Ma T, Tang M, Shen J, Ji H, Yang G (2020) Heterostructural composite of few-layered MoS2/hexagonal MoO2 particles/graphene as anode material for highly reversible lithium/sodium storage. Int J Energy Res 44(1):518–527
Sun M, Lan B, Lin T, Cheng G, Ye F, Yu L, Cheng X, Zheng X (2013) Controlled synthesis of nanostructured manganese oxide:crystalline evolution and catalytic activities. Cryst Eng Comm 15:7010–7018
Duan L, Sun B, Wei M, Luo S, Pan F, Xu A, Li X (2015) Catalytic degradation of Acid Orange 7 by manganese oxide octahedral molecular sieves with peroxymonosulfate under visible light irradiation. J Hazard Mater 285:356–365
Débart A, Paterson AJ, Bao J, Bruce PG (2008) α-MnO2 nanowires: a catalyst for the O2 electrode in rechargeable lithium batteries. Angew Chem Int Ed 47(24):4521–4524
Guo D, Yu X, Shi W, Luo Y, Li Q, Wang T (2014) Facile synthesis of well-ordered manganese oxide nanosheet arrays on carbon cloth for high-performance supercapacitors. J Mater Chem 2:8833–8838
Yin B, Zhang S, Jiao Y, Liu Y, Qu F, Wu X (2014) Facile synthesis of ultralong MnO2 nanowires as high performance supercapacitor electrodes and photocatalysts with enhanced photocatalytic activities. Cryst Eng Comm 16(43):9999–10005
Liu Q, Ishibashi A, Fujigaya T, Mimura K, Gotou T, Uera K, Nakashima N (2011) Formation of self-organized graphene honeycomb films on substrates. Carbon 49(11):3424–3429
Chang J, Zhou G, Christensen E, Heideman R, Chen J (2014) Graphene-based sensors for detection of heavy metals in water: a review. Anal Bioanal Chem 406:3957–3975
Shu J, Tang D (2019) Recent advances in photoelectrochemical sensing: from engineered photoactive materials to sensing devices and detection modes. Anal Chem 92(1):363–377
Ojani R, Safshekan S, Raoof JB (2014) Photoelectrochemical oxidation of hydrazine on TiO2 modified titanium electrode: its application for detection of hydrazine. J Solid State Electrochem 18(3):779–783
Zhang H, Gao Q, Li H (2016) A novel photoelectrochemical hydrogen peroxide sensor based on nickel (II)-potassium hexacyanoferrate-graphene hybrid materials modified n-silicon electrode. J Solid State Electrochem 20(6):1565–1573
Alves SA, Soares LL, Goulart LA, Mascaro LH (2016) Solvent effects on the photoelectrochemical properties of WO3 and its application as dopamine sensor. J Solid State Electrochem 20(9):2461–2470
Xu H, Huang D, Wu Y, Di J (2016) Photoelectrochemical determination of Cu2+ ions based on assembly of Au/ZnS nanoparticles. Sens Actuators, B Chem 235:432–438
Foo CY, Lim HN, Pandikumar A, Huang NM, Ng YH (2016) Utilization of reduced graphene oxide/cadmium sulfide-modified carbon cloth for visible-light-prompt photoelectrochemical sensor for copper (II) ions. J Hazard Mater 304:400–408
Liu Y, Li R, Gao P, Zhang Y, Ma H, Yang J, Du B, Wei Q (2015) A signal-off sandwich photoelectrochemical immunosensor using TiO2 coupled with CdS as the photoactive matrix and copper (II) ion as inhibitor. Biosens Bioelectron 65:97–102
Zhang D (2018) A novel signal-on photoelectrochemicalsensing platform based on biosynthesis of CdS quantum dots sensitizing ZnO nanorod arrays. Sens Actuators, B Chem 261:515–521
Wang H, Ye H, Zhang B, Zhao F, Zeng B (2017) Electrostatic interaction mechanism based synthesis of a Z-scheme BiOI−CdS photocatalyst for selective and sensitive detection of Cu2+. Journal of Materials Chemistry A 5(21):10599–10608
Huang F, Pu F, Lu X, Zhang H, Xia Y, Huang W, Li Z (2013) Photoelectrochemical sensing of Cu2+ ions with SnO2/CdS heterostructural films. Sens Actuators, B Chem 183:601–607
Wang J, Pan Y, Jiang L, Liu M, Liu F, Jia M, Lai Y (2019) Photoelectrochemical determination of Cu2+ using a WO3/CdS heterojunction photoanode. ACS Appl Mater Interfaces 11(41):37541–37549
Tang J, Li J, Zhang Y, Kong B, Yiliguma WY, QuanY CH, Al-Enizi AM, Gong X, Zheng G (2015) Mesoporous Fe2O3-CdS heterostructures for real-time photoelectrochemical dynamic probing of Cu2+. Anal Chem 87:6703–6708
Liang D, Liang X, Zhang Z, Wang H, Zhang N, Wang J, Qiu X (2020) A regenerative photoelectrochemical sensor based on functional porous carbon nitride for Cu2+ detection. Microche J 156:104922
Zeng H, Liu Y, Xu Z, Wang Y, Chai Y, Yuan R, Liu H (2019) Construction of a Zscheme gC3 N4/Ag/AgI heterojunction for highly selective photoelectrochemical detection of hydrogen sulfide. Chem Commun 55(79):11940–11943
Moulai F, Fellahi O, Messaoudi B, Hadjersi T, Zerroual L (2018) Electrodeposition of nanostructured γ-MnO2 film for photodegradation of Rhodamine B. Ionics 24(7):2099–2109
Bahrami A, Kazeminezhad I, Abdi Y (2019) Pt-Ni/rGO counter electrode: electrocatalytic activity for dye-sensitized solar cell. Superlattices Microstruct 125:125–137
Maghraoui-Meherzi H, Nasr TB, Dachraoui M (2013) Synthesis, structure and optical properties of Sb2Se3. Mater Sci Semicond Process 16(1):179–184
Pagnanelli F, Sambenedetto C, Furlani G, Vegliò F, Toro L (2007) Preparation and characterisation of chemical manganese dioxide: effect of the operating conditions. J Power Sources 166(2):567–577
Dubal DP, Dhawale DS, Gujar TP, Lokhande CD (2011) Effect of different modes of electrodeposition on supercapacitive properties of MnO2 thin films. Appl Surf Sci 257(8):3378–3382
Julien CM, Massot M, Poinsignon C (2004) Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochimica Acta Part A: Mole Biomole Spectros 60(3):689–700
Kumar NA, Gambarelli S, Duclairoir F, Bidan G, Dubois L (2013) Facile one-pot synthesis of graphene oxide by sonication assisted mechanochemical approach and its surface chemistry. J Mater Chem A1(8):2789–2794
Mondal J, Marandi M, Kozlova J, Merisalu M, Niilisk A, Sammelselg V (2014) Protection and functionalizing of stainless steel surface by graphene oxide-polypyrrole composite coating. J Chem Chem Eng 8:786–793
Yu X, Chen X, Ding X, Chen X, Yu X, Zhao X (2019) High-sensitivity and low hysteresis humidity sensor based on hydrothermally reduced grapheme oxide/nanodiamond. Sens Actuators, B Chem 283:761–768
Landolsi Z, Assaker IB, Nunes D, Fortunato E, Martins R, Chtourou R, Ammar S (2020) Enhanced electrical and photocatalytic properties of porous TiO2 thin films decorated with Fe2O3 nanoparticles. J Mater Sci: Mater Electron 31(23):20753–20773
Azani A, Halin DSC, Razak KA, Abdullah MMAB, Salleh MAAM, Mahmed N, Ramli MM, Sepeai S (2019) Microstructure and wettability of graphene oxide/TiO2 thin film prepared via sol-gel method. In IOP Conf Series: Mater Sci Eng 551(1):012099. IOP Publishing
Lamouchi A, Slimi B, Assaker IB, Gannouni M, Chtourou R (2016) Correlation between SSM substrate effect and physical properties of ZnO nanowires electrodeposited with or without seed layer for enhanced photoelectrochemical applications. The European Physical Journal Plus 131(6):1–11
Ahmad J, Wahid M, Majid K (2020) In situ construction of hybrid MnO2 @ GO heterostructures for enhanced visible light photocatalytic, anti-inflammatory and anti-oxidant activity. New J Chem 44(26):11092–11104
Lamouchi A, Assaker IB, Chtourou R (2019) Enhanced photoelectrochemical activity of MoS2-decorated ZnO nanowires electrodeposited onto stainless steel mesh for hydrogen production. Appl Surf Sci 478:937–945
Chen H, Zhou S, Chen M, Wu L (2012) Reduced graphene oxide–MnO2 hollow sphere hybrid nanostructures as high-performance electrochemical capacitors. J Mater Chem 22(48):25207–25216
Fei H, Saha N, Kazantseva N, Moucka R, Cheng Q, Saha P (2017) A highly flexible supercapacitor based on MnO2/RGO nanosheets and bacterial cellulose-filled gel electrolyte. Materials 10(11):1251
Zhu C, Guo S, Fang Y, Han L, Wang E, Dong S (2011) One-step electrochemical approach to the synthesis of graphene/MnO2 nanowall hybrids. Nano Res 4(7):648–657
Radhiyah AA, Izwan MI, Baiju V, Feng CK, Jamil I, Jose R (2015) Doubling of electrochemical parameters via the pre-intercalation of Na+ in layered MnO2 nanoflakes compared to α-MnO2 nanorods. RSC Adv 5(13):9667–9673
Misnon II, Jose R (2017) Synthesis and electrochemical evaluation of the PANI/δ-MnO2 electrode for high performing asymmetric supercapacitors. New J Chem 41(14):6574–6584
Hammami A, Raouafi N, Mirsky VM (2018) Electrically controlled Michael addition: addressing of covalent immobilization of biological receptors. Biosens Bioelectron 121:72–79
Kim TG, Ryu H, Lee WJ, Yoon JH (2015) Effects of graphene oxide (GO) on GO-Cu2O composite films grown by using electrochemical deposition for a PEC photoelectrode. J Korean Phys Soc 66(10):1586–1592
Yeh TF, Cihlář J, Chang CY, Cheng C, Teng H (2013) Roles of graphene oxide in photocatalytic water splitting. Mater Today 16(3):78–84
Merian E, Clarkson TW (1991) Metals and their compounds in the environment. Vch
Wang P, Huang D, Guo W, Di J (2018) Photoelectrochemical sensing for hydroquinone based on gold nanoparticle-modified indium tin oxide glass electrode. J Solid State Electrochem 22(1):123–128
Hammami A, Sahli R, Raouafi N (2016) Indirect amperometric sensing of dopamine using a redox-switchable naphthoquinone-terminated self-assembled monolayer on gold electrode. Microchim Acta 183(3):1137–1144
Funding
Authors would like to acknowledge the financial support of Tunisian Ministry of Higher Education and Scientific Research (PEJC19PEJC04-01 project (Programme d’Encouragement des Jeunes Chercheurs PEJC, 2ème Edition (2018))). Prof. Radhaoune Chtourou is also grateful for the financial support of Research and Technology Center of Energy (CRTEn), from Tunisia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hammami, A., Assaker, I.B. & Chtourou, R. Regenerative, low-cost and switchable photoelectrochemical sensor for detection of Cu2+ using MnO2-GO heterojunction. J Solid State Electrochem 26, 211–218 (2022). https://doi.org/10.1007/s10008-021-05092-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-05092-9