Abstract
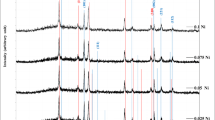
In this work, un-doped and Mn-doped TiO2 nanorod (NR) array films were successfully prepared by hydrothermal method that deposited on FTO substrate at different molar ratios (x = 0, 0.025, 0.05, 0.075, and 0.1). The prepared samples were examined by X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM). XRD illustrates the pure rutile phase in all samples. The preferred orientation along [001] for pure sample convert to [101] direction with increasing doping content. Lattice constants increase with increasing doping content. The top view of FESEM images show the uniformly distributed nanorod arrays. The nanorods’ length decrease, and their diameters increase with increasing doping content. The nanorods start to bevel from its vertical direction after doping. The crystal size of TiO2 NRs with different manganese doping has calculated using the Williamson-Hall plot. The saturation magnetism appeared in pure titanium oxide has been attributed to the oxygen vacancies, while it changed to diluted magnetic semiconductor after doped with Mn.








Similar content being viewed by others
References
Rafiq M, Nafees M (2018) Structural optical and magnetic study of Ni- doped TiO2 nanoparticles synthesized by sol – gel method. Int Nano Lett 8:1–8
Kalb J, Dorman J, Siroky S, Schmidt-Mende L (2019) Controlling the spatial direction of hydrothermally grown rutile TiO2 nanocrystals by the orientation of seed crystals. Crystals 9:1–8
Noman M, Ashraf M, Ali A (2019) Synthesis and applications of nano-TiO2: a review. Environ Sci Pollut Res 26(4):3262–3291
Barka N, Assabbane A (2008) Photocatalytic degradation of methyl orange with immobilized TiO2 nanoparticles: effect of pH and some inorganic anions. Phys Chem News 41:85–88
Salman ON, Agool IR, Ismail MM (2017) Preparation of the scattering layer based on TiO2 nanotube and their dye sensitized solar cell applications. Appl Phys A Mater Sci Process 123(6):402
Bupasiri T, Tunlasakun K, Mungkung N (2017) Characteristics of TiO2 nano structure electrode layer electrochromic device (smart window application), in international conference on applied electrical and mechanical engineering 2017:198–202
Feng T, Feng G, Yan L, Pan J (2014) One-dimensional nanostructured TiO2 for photocatalytic degradation of organic pollutants in wastewater. Int J Photoenergy 2014:1–14
AL-Jawad SMH, Salman ON, Yousif NA (2019) Structural, optical and electrical properties of TiO2 NR arrays deposited by hydrothermal method. Surf Rev Lett 26(03):1850155
AL-Jawad SMH, Mohammad RM, Imran NJ (2018) Effect of electrolyte solution on structural and optical properties of TiO2 grown by anodization technique for photoelectrocatalytic application. Surf Rev Lett 25(04):1850078
Iraj M, Nayeri F, Asl-soleimani E, Narimani K (2015) Controlled growth of vertically aligned TiO2 nanorod arrays using the improved hydrothermal method and their application to dye-sensitized solar cells. J Alloys Compd 20:1–7
Fajariah N, Prabowo W, Fathurrahman F, Melati A, Dipojono KH (2017) The investigation of electronic structure of transition metal doped TiO2 for diluted magnetic semiconductor applications: a first principle study. Procedia Eng 170:41–147
Hong N, Sakai J, Prellier W, Antoine R (2004) Ferromagnetism in transition-metal-doped Ti O2 thin films. Phys Rev 70:1–6
Chen L, Tian J, Qiu H, Yin Y, Wang X, Dai J, Wu P, Wang A, Chu L (2009) Preparation of TiO2nanofilm via sol-gel process and its photocatalytic activity for degradation of methyl orange. Ceram Int 35(8):3275–3280
Attar A, Mirdamadi S, Hajiesmaeilbaigi F, Ghamsari M (2007) Growth of TiO2 nanorods by sol – gel template process growth of TiO2 nanorods by sol-gel template process. J Mater Sci Technol 23(5):1–4
Pradhan S, Reucroft P, Yang F, Dozier A (2003) Growth of TiO2 nanorods by metalorganic chemical vapor deposition. J Cryst Growth 256(1–2):83–88
Li J, Ridge O, Wu J (2016) Synthesis of nanoparticles via solvothermal and hydrothermal methods. Handb Nanoparticles 17:1–28
AL-Jawad SMH, Taha A, Redha A (2019) Studying the structural, morphological, and optical properties of CuS:Ni nanostructure prepared by a hydrothermal method for biological activity. J Sol-Gel Sci Technol 91(2):310–323
AL-Jawad SMH, Ismail MM, Emad S (2017) Characterization of Mn, cu, and (Mn, cu) co-doped ZnS nanoparticles. J Opt Tech 84(7):80–85
Burungale V, Satale V, Teli A, Kamble A, Kim J, Patil P (2016) Surfactant free single step synthesis of TiO2 3-D micro flowers by hydrothermal route and its photoelectrochemical characterizations. J Alloys Compd 656:491–499
Zheng Z, Chen J, Yoshida R, Gao X, Tarr K (2014) One-step synthesis of TiO2 nanorod arrays on Ti foil for supercapacitor application. Nanotechnology 25(43):1–7
Jithin M, Saravanakumar K, Ganesan V, Reddy V, Razad P, Patidar M, Jeyadheepan K, Marimuthu G, Sreelakshmi V, Mahalakshmi K (2017) Growth, mechanism and properties of TiO2 nanorods embedded nanopillar: evidence of lattice orientation effect. Superlattice Microst 109:145–153
Liu S, Plawsky J (2017) Solid-state dewetting of gold aggregates/islands on TiO2 nanorod structures grown by oblique angle deposition. Langmuir 33(49):14066–14077
Al-jawad SMH (2017) Comparative study between CBD and SILAR methods for deposited TiO2, CdS, and TiO2 / CdS core-shell structure. Mater Sci Semicond Process 67:75–83
Diaz R, Bihri H, Aouaj M, Rueda F (2007) Preparation and characterization of sprayed FTO thin films. Eur Phys J Appl Phys 219:217–219
Meagher L (1979) Polyhedral thermal expansion in the TiO2 polymorphs: refinement of the crystal structure of rutile and brookite at high temperature sample at 300 degrees C. Can Mineral 17:77–85
Hassanzadeh A, Moazzez B, Haghgooie H, Nasseri M, Golzan M, Sedghi H (2008) Synthesis of SnO2 nanopowders by a sol-gel process using propanol-isopropanol mixture. Cent Eur J Chem 6(4):651–656
Zhang S (2017) Study of fluorine-doped tin oxide (FTO) thin films for photovoltaics applications. PH. D. Thesis, Materials. Université Grenoble Alpes
Sellers M, Seebauer E (2013) Room temperature ferromagnetism in Mn-doped TiO2 nanopillar matrices. Mater Lett 20:1–15
Al-Jawad SMH, Elttayf A, Saber A (2017) Studying structural, optical, electrical, and sensing properties of nanocrystalline SnO2:cu films prepared by sol-gel method for CO gas sensor application at low temperature. Surf Rev Lett 24(8):1–12
Delhez R, Mittemeijer E (1982) Determination of crystallite size and lattice distortions through X-ray diffraction line profile analysis. Fresenius Z Anal Chem 312(1):1–16
Gokul B, Matheswaran P, Sathyamoorthy R (2013) Influence of annealing on physical properties of CdO thin films prepared by SILAR method. J Mater Sci Technol 29(1):17–21
Mukhtar M (2012) Co-precipitation synthesis and characterization of nanocrystalline zinc oxide particles doped with Cu2+ ions. Mater Sci Appl 3(08):543–551
Ni S, Guo F, Wang D, Jiao S (2019) Modification of TiO2 nanowire arrays with Sn doping as photoanode for highly efficient. Crystals 9(2):113–125
Galstyan V (2017) Porous TiO2 -based gas sensors for cyber chemical systems to provide security and medical diagnosis. Sensors 17(12):1–25
Chan S, Li M, Wei H, Chen S, Kuo C (2015) The effect of annealing on nanothick indium tin oxide transparent conductive films for touch sensors. J Nanomater 2015:1–5
Sapra S, Sarma D, Sanvito S, Hill N (2002) Influence of quantum confinement on the electronic and magnetic properties of (Ga,Mn) as diluted magnetic semiconductor. Nano Lett 2(6):605–608
SMH AL-J, Rafic SN, Muhsen MM (2017) Preparation and characterization of polyaniline-cadmium sulfide nanocomposite for gas sensor application. Mod Phys Lett B 31(26):1750234
AL-Jawad SMH (2017) Structural and optical properties of core–shell TiO2/CdS prepared by chemical bath deposition. J Electron Mater 46(10):5837–5847
Kaminski A, Das Sarma S (2002) Polaron percolation in diluted magnetic semiconductors. Phys Rev Lett 88(24):247202
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Jawad, S.M.H., Ismail, M.M. & Ghazi, S.F. Characteristics of diluted magnetic semiconductor based on Mn-doped TiO2 nanorod array films. J Solid State Electrochem 25, 435–443 (2021). https://doi.org/10.1007/s10008-020-04823-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04823-8