Abstract
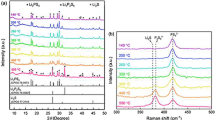
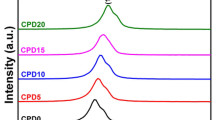
Pr0.2Ce0.8O2-δ@Li2CO3 (PDC-LC) nanocomposite electrolytes were prepared through the co-precipitation of Pr-doped cerium/lithium complex carbonate and its pre-firing and sintering operations. The structural and thermal properties of PDC-LC nanocomposite were investigated by means of X-ray diffraction, thermogravimetric analysis, Raman spectroscopy, and electrochemical impedance spectroscopy. The remarkable presence of oxygen vacancies and the influence of the interfacial interactions between PDC crystallites and amorphous Li2CO3 were deliberately probed by Raman spectroscopy and FEG-SEM, respectively. It was observed that there is a long-range interaction between the PDC crystallites and Li2CO3 phase, which resulted in the tight binding of \( {\mathrm{CO}}_3^{2-} \) ions on the facets of PDC nano-crystals and may be well characterized by the shift and broadening of the characteristic Raman spectroscopic peaks of \( {\mathrm{CO}}_3^{2-} \) ions in the samples. The X-ray diffraction results confirm the successful incorporation of praseodymium into ceria phase that presented the sole crystalline structure contrarily to the lithium carbonate present as an amorphous phase in the as-prepared samples. The oxygen ionic conductivity behaviors of PDC-LC phase were measured at the intermediate operating temperature range (300–500 °C). However, AC conductivity measurements demonstrated that the conductivities in air atmosphere increased linearly with temperature. The Arrhenius plot of total conductivity showed two different slopes indicating two distinct conduction mechanisms. The interfacial interactions between the PDC and LC phase inside the PDC-LC nanocomposite were responsible for oxygen ionic conductivity.









Similar content being viewed by others
References
Chroneos A, Yildiz B, Tarancón A, Parfitt D, Kilner JA (2011) Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: mechanistic insights from atomistic simulations. Energy Environ Sci 4(8):2774–2789
Wachsman ED, Lee KT (2011) Lowering the temperature of solid oxide fuel cells. Science 334(6058):935–939
Zhang Y, Knibbe R, Sunarso J, Zhong Y, Zhou W, Shao Z, Zhu Z (2017) Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv Mater 29(48):1–33
Duan C, Tong J, Shang M, Nikodemski S, Sanders M, Ricote S, Almansoori A, O'Hayre R (2015) Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349(6254):1–6
Gao Z, Mogni LV, Miller EC, Railsback JG, Barnett SA (2016) A perspective on low-temperature solid oxide fuel cells. Energy Environ Sci 9(5):1602–1644
Leng YJ, Chan SH, Khor KA, Jiang SP (2004) Performance evaluation of anode-supported solid oxide fuel cells with thin film YSZ electrolyte. Int J Hydrog Energy 29(10):1025–1033
Liu J, Barnett SA (2003) Operation of anode-supported solid oxide fuel cells on methane and natural gas. J Solid State Ionics 158(1–2):11–16
Hui S, Roller J, Yick S, Zhang X, Deces-Petit C, Xie Y, Maric R, Ghosh D (2007) A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J Power Sources 172(2):493–502
Singhal SC, Kendall K (2003) High-temperature solid oxide fuel cells: fundamentals, design and applications. Elsevier Science Ltd, pp 1–406. https://doi.org/10.1016/B978-1-85617-387-2.X5016-8
Butz B, Kruse P, Stormer H, Gerthsen D, Muller A, Weber A, Ivers-Tiffee E (2006) Correlation between microstructure and degradation in conductivity for cubic Y2O3-doped ZrO2. Solid State Ionics 177(37–38):3275–3284
Benamira M, Ringuedé A, Albin V, Vannier R-N, Hildebrandt L, Lagergren C, Cassir M (2011) Gadolinia-doped ceria mixed with alkali carbonates for solid oxide fuel cell applications: I. A thermal, structural and morphological insight. J Power Sources 196(13):5546–5554
Chockalingam R, Basu S (2016) Impedance spectroscopy studies of Gd-CeO2-(LiNa)CO3 nanocomposite electrolytes for low temperature SOFC application. Int J Hydrog Energy 36(22):14977–14983
Huang J, Gao Z, Mao Z (2010) Effects of salt composition on the electrical properties of samaria-doped ceria/carbonate composite electrolytes for low-temperature SOFCs. Int J Hydrog Energy 35(9):4270–4275
Gao Z, Raza R, Zhu B, Mao Z, Wang C, Liu Z (2011) Preparation and characterization of Sm0.2Ce0.8O1.9/Na2CO3nanocomposite electrolyte for low-temperature solid oxide fuel cells. Int J Hydrog Energy 36(6):3984–3988
Di J, Chen M, Wang C, Zheng J, Fan L, Zhu B (2010) Samarium doped ceria–(Li/Na)2CO3 composite electrolyte and its electrochemical properties in low temperature solid oxide fuel cell. J Power Sources 195(15):4695–4699
Di J, Chen MM, Wang CY, Zheng JM, Zhu B (2010) Low temperature solid oxide fuel cells with SDC-carbonate electrolytes. Adv Mater Res 105:687–690
Tang Z, Lin Q, Mellander B, Zhu B (2010) SDC–LiNa carbonate composite and nanocomposite electrolytes. Int J Hydrog Energy 35(7):2970–2975
Cai T, Zeng Y, Yin S, Wang L, Li C (2011) Preparation and characterization of Ce0.8Sm0.2O1.9(SDC)-carbonates composite electrolyte via molten salt infiltration. Mater Lett 65(17):2751–2754
Ma Y, Wang X, Raza R, Muhammed M, Zhu B (2010) Thermal stability study of SDC/Na2CO3 nanocomposite electrolyte for low-temperature SOFCs. Int J Hydrog Energy 35(7):2580–2585
Zhang L, Lan R, Xu X, Tao S, Jiang Y, Kraft A (2009) A high performance intermediate temperature fuel cell based on a thick oxide–carbonate electrolyte. J Power Sources 194(2):967–971
Liu W, Liu Y, Li B, Sparks TD, Wei X, Pan W (2010) Ceria (Sm3+, Nd3+)/carbonates composite electrolytes with high electrical conductivity at low temperature. Compos Sci Technol 70(1):181–185
Raza R, Wang X, Ma Y, Zhu B (2010) Study on calcium and samarium co-doped ceria based nanocomposite electrolytes. J Power Sources 195(19):6491–6495
Rafique A, Raza R, Akram N, Ullah MK, Ali A, Irshad M, Siraj K, Khan MA, Zhu B, Dawson R (2015) Significance enhancement in the conductivity of core shell nanocomposite electrolytes. RSC Adv 5(105):86322–86329
Fan L, Zhang G, Chen M, Wang C, Di J, Zhu B (2012) Proton and oxygen ionic conductivity of doped ceria-carbonate composite by modified wagner polarization. Int J Electrochem Sci 7(9):8420–8435
Zhu B (2003) Functional ceria-salt-composite materials for advanced ITSOFC applications. J Power Sources 114(1):1–9
Fan L, Wang C, Chen M, Zhu B (2013) Recent development of ceria-based (nano)composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J Power Sources 234:154–174
Xia C, Li Y, Tian Y, Liu Q, Zhao Y, Jia L, Li Y (2009) A high performance composite ionic conducting electrolyte for intermediate temperature fuel cell and evidence for ternary ionic conduction. J Power Sources 188(1):156–162
Zhao Y, Xia C, Jia L, Wang Z, Li H, Yu J, Li Y (2013) Recent progress on solid oxide fuel cell: Lowering temperature and utilizing non-hydrogen fuels. Int J Hydrog Energy 38(36):16498–16517
Rietveld H (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2(2):65–71
McCusker LB, Von Dreele RB, Cox DE, Louër D, Scardi P (1999) Rietveld refinement guidelines. J Appl Crystallogr 32:36–50
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. J Phys B Condens Matter 192(1–2):55–69
Berar J-F, Baldinozzi G (1993) Modeling of line-shape asymmetry in powder diffraction. J Appl Crystallogr 26(1):128–129
Dastan D (2017) Effect of preparation methods on the properties of titania nanoparticles: solvothermal versus sol-gel. Appl Phys A Mater Sci Process 123(699):1–13
Li C, Zeng Y, Wang Z, Ye Z, Zhang Y (2017) Processing temperature tuned interfacial microstructure and protonic and oxide ionic conductivities of well-sintered Sm0.2Ce0.8O1.9- Na2CO3 nanocomposite electrolytes for intermediate temperature solid oxide fuel cells. J Power Sources 360:114–123
Yin S, Zeng Y, Li C, Chen X, Ye Z (2013) Investigation of Sm0.2Ce0.8O1.9/Na2CO3 nanocomposite electrolytes: preparation, interfacial microstructures, and ionic conductivities. ACS Appl Mater Interfaces 5(24):12876–12886
Li C, Zeng Y, Wang Z, Ye Z, Zhang Y, Shi R (2016) Preparation of SDC–NC nanocomposite electrolytes with elevated densities: influence of prefiring and sintering treatments on their microstructures and electrical conductivities. RSC Adv 6(102):99615–99624
Dastan D, Londhe PU, Chaure NB (2014) Characterization of TiO2 nanoparticles prepared using different surfactants by sol-gel method. J Mater Sci Mater Electron 25(8):3473–3479
Dastan D, Panahi SL, Yengntiwar AP, Banpurkar AG (2016) Morphological and electrical studies of titania powder and films grown by aqueous solution method. Adv Sci Lett 22(4):950–953
Dastan D, Chaure N, Kartha M (2017) Surfactants assisted solvothermal derived titania nanoparticles: synthesis and simulation. J Mater Sci Mater Electron 28(11):7784–7796
Dastan D, Panahi SL, Chaure NB (2016) Characterization of titania thin films grown by dip-coating technique. J Mater Sci Mater Electron 27(12):12291–12296
Ţălu Ş (2015) Micro and nanoscale characterization of three dimensional surfaces: basics and applications. Napoca Star Publishing House, Cluj-Napoca
Dastan D, Chaure NB (2014) Influence of surfactants on TiO2 nanoparticles grown by Sol-Gel Technique. J Mater Mech Manufact 2(1):21–24
Zhao Y, Xu Z, Xia C, Li Y (2013) Oxide ion and proton conduction in doped ceria–carbonate composite materials. Int J Hydrog Energy 38(3):1553–1559
Muhammed Ali SA, Rosli RE, Muchtar A, Sulong AB, Somalu MR, Majlan EH (2015) Effect of sintering temperature on surface morphology and electrical properties of samarium-doped ceria carbonate for solid oxide fuel cells. J Ceram Int 41(1):1323–1332
Dastan D, Banpurkar A (2017) Solution processable sol-gel derived titania gate dielectric for organic field effect transistors. J Mater Sci Mater Electron 28(4):3851–3859
Uthayakumar A, Pandiyan A, Mathiyalagan S, Kumar A, Keshri AK, Omar S, Balani K, Moorthy SBK (2016) Interfacial effect of the oxygen-ion distribution on the conduction mechanism in strontium-added Ce0.8Sm0.2O2−δ/Na2CO3 nanocomposite. J Phys Chem C 120(43):25068–25077
Dastan D (2015) Nanostructured anatase titania thin films prepared by sol-gel dip coating technique. J. Atomic, Molecul. Condensate Nano Phys 2(2):109–114
Panahi SL, Dastan D, Chaure NB (2016) Characterization of zirconia nanoparticles grown by sol–gel method. Adv Sci Lett 22(4):941–944
Esther Jeyanthi C, Siddheswaran R, Kumar P, Chinnu MK, Rajarajan K, Jayavel R (2015) Investigation on synthesis, structure, morphology, spectroscopic and electrochemical studies of praseodymium-doped ceria nanoparticles by combustion method. J Mater Chem Phys 15:22–28
Ahn K, Yoo DS, Prasad DH, Lee H, Chung Y, Lee J (2012) Role of multivalent Pr in the formation and migration of oxygen vacancy in Pr-doped ceria: experimental and first-principles investigations. J Chem Mater 24(21):4261–4267
McBride JR, Hass KC, Pointdexter BD, Weber WH (1994) Raman and x-ray studies of Ce1−xRExO2−y, where RE=La, Pr, Nd, Eu, Gd, and Tb. J Appl Phys 76(4):2435–2441
Mineshige A, Taji T, Muroi Y, Kobune M, Fujii S, Nishi N, Inaba M, Ogumi Z (2000) Oxygen chemical potential variation in ceria-based solid oxide fuel cells determined by Raman spectroscopy. J Solid State Ionics 135(1–4):481–485
Paunović N, Dohčević-Mitrović Z, Curtu R, Aškrabić S, Prekajski M, Matović B, Popović ZV (2012) Suppression of inherent ferromagnetism in Pr-doped CeO2 nanocrystals. Nanoscale 4(17):5469–5476
Shajahan I, Ahn J, Nair P, Medisetti S, Patil S, Niveditha V, Uday Bhaskar Babu G, Prasad Dasari H, Lee J-H (2018) Praseodymium doped ceria as electrolyte material for IT-SOFC applications. J Mater Chem Phys 216:136–142
Anwar MS, Kumar S, Ahmed F, Arshi N, Seo YJ, Lee CG, Koo BH (2011) Study of nanocrystalline ceria thin films deposited by e-beam technique. Curr Appl Phys 11(11):301–304
Brooker MH, Bates JB (1971) Raman and infrared spectral studies of anhydrous Li2CO3 and Na2CO3. Chem Phys 54(11):4788–4796
Friesen G, Ozsar ME, Dunlop E (n.d.) Impedance model for CdTe solar cells exhibiting constant phase element behavior. Thin Solid Films 361–362:303–308
Pant M, Kanchan DK, Sharma P, Jayswal MS (2008) Mixed conductivity studies in silver oxide based barium vanado–tellurite glasses. Mater Sci Eng B 149(1):18–25
Jonscher AK (1977) The ‘universal’ dielectric response. Nature 267:673–679
Dastan D, Gosavi WS, Chaure BN (2015) Studies on electrical properties of hybrid polymeric gate dielectric for field effect transistors. Macromol Symp 347:81–86
Midouni A, Houchati MI, Belhaj Othman W, Chniba-Boudjada N, Jaouadi M, Ceretti M, Paulus W, Hamzaoui AH (2016) Influence of nickel doping on oxygen-ionic conductivity of the n = 1 Ruddlesden-Popper phases La1.85Ca0.15(Cu1−xNix)O4−δ (δ = 0.0905). J Solid State Chem 240:101–108
Aarthi U, Arunkumar P, Sribalaji M, Kumar Keshrib A, Suresh Babu K (2016) Strontium mediated modification of structure and ionic conductivity in samarium doped ceria/sodium carbonate nanocomposites as electrolytes for LTSOFC. RSC Adv 6(88):84860–84870
Rahmawati F, Nuryanto A, Nugrahaningtyas KD (2016) The single cell of low temperature solid oxide fuel cell with sodium carbonate-SDC (samarium-doped ceria) as electrolyte and biodiesel as fuel. IOP Conference Series: Materials Science and Engineering, vol 107, p 012035
Ali A, Rafique A, Kaleemullah M, Abbas G, Khan MA, Ahmad MA, Raza R (2018) Effect of alkali carbonates (single, binary, and ternary) on doped ceria: a composite electrolyte for low-temperature solid oxide fuel cells. ACS Appl Mater Interfaces 10(1):806–818
Maheshwari A, Wiemhofer HD (2016) Augmentation of grain boundary conductivity in Ca2+ doped ceria-carbonate-composite. Acta Mater 103:361–369
Lapa CM, Figueiredo FML, de Souza DPF, Song L, Zhu B, Marques FMB (2010) Synthesis and characterization of composite electrolytes based on samaria-doped ceria and Na/Li carbonates. Int J Hydrog Energy 35(7):2953–2957
Ristoiu T, Petrisor T, Gabor M, Rada S, Popa F, Ciontea L, Traian P (2012) Electrical properties of ceria/carbonate nanocomposites. J Alloys Compd 532:109–113
Sadykov VA, Kuznetsova TG, Frolova-Borchert Yu V, Alikina GM, Lukashevich AI, Rogov VA, Muzykantov VS, Pinaeva LG, Sadovskaya EM, Ivanova Yu A, Paukshtis EA, Mezentseva NV, Batuev LC, Parmon VN, Neophytides S, Kemnitz E, Scheurell K, Mirodatos C, van Veen AC (2006) Fuel-rich methane combustion: role of the Pt dispersion and oxygen mobility in a fluorite-like complex oxide support. Catal Today 117(4):475–483
Spiridigliozzi L, Dell'Agli G, Accardo G, Yoon SP, Frattini D (2019) Electro-morphological, structural, thermal and ionic conduction properties of Gd/Pr co-doped ceria electrolytes exhibiting mixed Pr3+/Pr4+ cations. Ceram Int 45(4):4570–4580
Funding
Financial support was received from the EMORI MOBIDOC (Project funded by the European Union) Postdoctoral Research Fellowship Program from national agency for the promotion of scientific research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Midouni, A., Houchati, M.I., Selmi, W. et al. Investigation of Pr0.2Ce0.8O2-δ@Li2CO3 nanocomposite electrolytes as intermediate temperature ionic conductors: a thermal, structural, and morphological insight. J Solid State Electrochem 23, 2465–2475 (2019). https://doi.org/10.1007/s10008-019-04338-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04338-x