Abstract
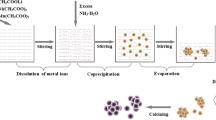
Lithium-rich layered oxide Li[Li0.23Ni0.15Mn0.62]O2, which also can be written as 0.6Li2MnO3·0.4LiNi0.5Mn0.5O2 or 0.9Li[Li1/3Mn2/3]O2·0.4LiNi0.5Mn0.5O2, is synthesized using a solid-state reaction method. Its crystal structure and electrochemical behavior as the cathode material in lithium-ion batteries are studied. A reaction mechanism is proposed to interpret its unique electrochemical behavior shown in the first charge–discharge cycle. It includes four reactions: (1) LiNi0.5Mn0.5O2 → Li+ + Ni0.5Mn0.5O2 + e−, (2) Li[Li1/3Mn2/3]O2 → Li+ + [Li1/3Mn2/3]O2 + e−, (3) [Li1/3Mn2/3]O2 → 1/3 Li+ + 2/3 MnO2 + 2/3 O· + e−, and (4) Li+ + Ni0.2Mn0.8O2 + e− → LiNi0.2Mn0.8O2. The extraction of oxygen atoms (O·) in the reaction (3) results in the crystal structure rearrangement. Based on this hypothesis, it is found that the expected capacity of activated lithium-rich layered oxide xLi2MnO3·(1 − x)LiNi0.5Mn0.5O2 (0 ≤ x ≤ 1) increases from 230 to 280 mAh g−1 with increasing x value. Li[Li0.23Ni0.15Mn0.62]O2 has an expected total first charge capacity of 396 mAh g−1, but its expected capacity is only 247 mAh g−1 due to an initial capacity loss caused by the oxygen loss. Experimentally, within a charge–discharge voltage window from 2.0 to 4.8 V, Li[Li0.23Ni0.15Mn0.62]O2 delivers a charge capacity of 310 mAh g−1 and a discharge capacity of 215 mAh g−1, respectively, at 40 mA g−1 during the first cycle. The electrochemical kinetic behavior of Li[Li0.23Ni0.15Mn0.62]O2 is controlled by the charge-transfer process rather than by Li+ diffusion or blockage of solid-electrolyte interphase (SEI) layers at the end of Li+ extraction in the first charge.












Similar content being viewed by others
References
Goodenough JB (2007) Cathode materials: a personal perspective. J Power Sources 174:996–1000
Brodd RJ, Bullock KR, Leising RA, Middaugh RL, Miller JR, Takeuchi E (2004) Batteries, 1977 to 2002. J Electrochem Soc 151:K1–K11
Cho J, Park B (2001) Preparation and electrochemical thermal properties of LiNi0.74Co0.26O2 cathode material. J Power Sources 92:35–39
Yabuuchi N, Ohzuku T (2003) Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J Power Sources 119–121:171–174
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Lu Z, MacNeil DD, Dahn JR (2001) Layered cathode materials Li[Ni x Li1/3−2x/3]Mn2/3−x/3O2 for lithium-ion batteries. Electrochem Solid-State Lett 4:A191–A194
Kang SH, Kempgens P, Greenbaum S, Kropf AJ, Amine K, Thackeray MM (2007) Interpreting the structural and electrochemical complexity of 0.5Li2MnO3 · 0.5LiMO2 electrodes for lithium batteries (M = Mn0.5 − xNi0.5 − xCo2x, 0x0.5). J Mater Chem 17:2069–2077
Johnson CS, Li N, Lefief C, Vaughey JT, Thackeray MM (2008) Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3 · (1 − x)LiMn0.333Ni0.333Co0.333O2 (0x0.7). Chem Mater 20:6095–6106
Yabuuchi N, Yoshii K, Myung ST, Nakai I, Komaba S (2011) Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3–LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133:4404–4419
Kim S, Kim C, Jhon YI, Noh JK, Vemuri SH, Smith R, Chung KY, Jhon MS, Cho BW (2012) Synthesis of layered–layered 0.5Li2MnO3 · 0.5LiCoO2 nanocomposite electrode materials by the mechanochemical process and first principles study. J Mater Chem 22:25418–25426
Neudecker BJ, Zuhr RA, Kwak BS, Bates JB, Robertson JD (1998) Lithium manganese nickel oxides Li x (Mn y Ni1–y )2–x O2 I. Synthesie and characterization of thin films and bulk phases. J Electrochem Sci 145:4148–4159
Kim D, Gim J, Lim J, Park S, Kim J (2010) Synthesis of xLi2MnO3 · (1–x)LiMO2 (M = Cr, Mn, Co, Ni) nanocomposites and their electrochemical properties. Mater Res Bull 45:252–255
Jarvis KA, Deng Z, Allard LF, Manthiram A, Ferreira PJ (2011) Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: evidence of a solid solution. Chem Mater 23:3614–3621
Wang CC, Jarvis KA, Ferreira PJ, Manthiram A (2013) Effect of synthesis conditions on the first charge and reversible capacities of lithium-rich layered oxide cathodes. Chem Mater 25:3267–3275
Lu Z, Dahn JR (2002) Understanding the anomalous capacity of Li/Li[Ni x Li1/3−2x/3Mn2/3−x/3]O2 cells using in situ X-ray diffraction and electrochemical studies. J Electrochem Soc 149:A815–A822
Armstrong AR, Holzapfel M, Novak P, Johnson CS, Kang SH, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J Am Chem Soc 128:8694–8698
Jiang M, Key B, Meng YS, Grey CP (2009) Electrochemical and structural study of the layered, “Li-excess” lithium-ion battery electrode material Li[Li1/9Ni1/3Mn5/9]O2. Chem Mater 21:2733–2745
Boulineau A, Simonin L, Colin JF, Bourbon C, Patoux S (2013) First evidence of manganese-nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett 13:3857–3863
Zhang J, Guo X, Yao S, Zhu W, Qiu X (2013) Tailored synthesis of Ni0.25Mn0.75CO3 spherical precursors for high capacity Li-rich cathode materials via a urea-based precipitation method. J Power Sources 238:245–250
Wang ZH, Yuan LX, Zhang WX, Huang YH (2012) LiFe0.8Mn0.2PO4/C cathode material with high energy density for lithium-ion batteries. J Alloys Compd 532:25–30
Ohzuku T, Nagayama M, Tsuji K, Ariyoshi K (2011) High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: toward rechargeable capacity more than 300 mA h g−1. J Mater Chem 21:10179–10188
Kang SH, Thackeray MM (2009) Enhancing the rate capability of high capacity xLi2MnO3 · (1 − x) LiMO2 (M = Mn, Ni, Co) electrodes by Li–Ni–PO4 treatment. Electrochem Commun 11:748–751
Abraham DP, Twesten RD, Balasubramanian M, Petrov I, McBreen J, Amine K (2002) Surface changes on LiNi0.8Co0.2O2 particles during testing of high-power lithium-ion cells. Electrochem Commun 4:620–625
Lu Z, Beaulieu LY, Donaberger RA, Thomas CL, Dahn JR (2002) Synthesis, structure, and electrochemical behavior of Li[Ni x Li1/3−2x/3Mn2/3−x/3]O2. J Electrochem Soc 149:A778–A791
Yang L, Cheng X, Ma Y, Lou S, Cui Y, Guan T, Yin G (2013) Changing of SEI film and electrochemical properties about MCMB electrodes during long-term charge/discharge cycles. J Electrochem Soc 160:A2093–A2099
Cherkashinin G, Nikolowski K, Ehrenberg H, Jacke S, Dimesso L, Jaegermann W (2012) The stability of the SEI layer, surface composition and the oxidation state of transition metals at the electrolyte–cathode interface impacted by the electrochemical cycling: X-ray photoelectron spectroscopy investigation. Phys Chem Chem Phys 14:12321–12331
Lim J, Moon J, Gim J, Kim S, Kim K, Song J, Kang J, Im WB, Kim J (2012) Fully activated Li2MnO3 nanoparticles by oxidation reaction. J Mater Chem 2012(22):11772–11777
Amalraj SF, Sharon D, Talianker M, Julien CM, Burlaka L, Lavi R, Zhecheva E, Markovsky B, Zinigrad E, Kovacheva D, Stoyanova R, Aurbach D (2013) Study of the nanosized Li2MnO3: electrochemical behavior, structure, magnetic properties, and vibrational modes. Electrochim Acta 97:259–270
Robertson AD, Bruce PG (2003) Mechanism of electrochemical activity in Li2MnO3. Chem Mater 15:1984–1992
Li D, Muta T, Noguchi H (2004) Electrochemical characteristics of LiNi0.5Mn0.5−x Ti x O2 prepared by solid state method. J Power Sources 135:262–266
Manikandan P, Ananth MV, Kumar TP, Raju M, Periasamy P, Manimaran K (2011) Solution combustion synthesis of layered LiNi0.5Mn0.5O2 and its characterization as cathode material for lithium-ion cells. J Power Sources 196:10148–10155
Liu Y, Cao F, Chen B, Zhao X, Suib SL, Chan HLW, Yuan J (2012) High performance of LiNi0.5Mn0.5O2 positive electrode boosted by ordered three-dimensional nanostructures. J Power Sources 206:230–235
Kumar PS, Sakunthala A, Prabub M, Reddy MV, Joshi R (2014) Structure and electrical properties of lithium nickel manganese oxide (LiNi0.5Mn0.5O2) prepared by P123 assisted hydrothermal route. Solid State Ionics 267:1–8
Tan S, Zhang Z, Li Y, Li Y, Zheng J, Zhou Z, Yang Y (2013) Tris(hexafluoro-iso-propyl)phosphate as an SEI-forming additive on improving the electrochemical performance of the Li[Li0.2Mn0.56Ni0.16Co0.08]O2 cathode material. J Electrochem Soc 160:A285–A292
Kang YS, Yoon T, Lee SS, Mun J, Park MS, Park JH, Doo SG, Song IY, Oh SM (2013) 1,3,5-Trihydroxybenzene as a film-forming additive for high-voltage positive electrode. Electrochem Commun 27:26–28
Li Z, Du F, Bie X, Zhang D, Cai Y, Cui X, Wang C, Chen G, Wei Y (2010) Electrochemical kinetics of the Li[Li0.23Co0.3Mn0.47]O2 cathode material studied by GITT and EIS. J Phys Chem C 114:22751–22757
Amalraj F, Kovacheva D, Talianker M, Zeiri L, Grinblat J, Leifer N, Goobes G, Markovsky B, Aurbach D (2010) Synthesis of integrated cathode materials xLi2MnO3 · xLi2MnO3 · (1 − x)LiMn1/3Ni1/3Co1/3O2 (x = 0.3, 0.5, 0.7) and studies of their electrochemical behavior. J Electrochem Soc 157:A1121–A1130
Gao P, Yang G, Liu H, Wang L, Zhou H (2012) Lithium diffusion behavior and improved high rate capacity of LiNi1/3Co1/3Mn1/3O2 as cathode material for lithium batteries. Solid State Ionics 207:50–56
Jafta CJ, Ozoemena KI, Mathe MK, Roos WD (2012) Synthesis, characterisation and electrochemical intercalation kinetics of nanostructured aluminium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion battery. Electrochim Acta 85:411–422
Yan SY, Wang CY, Gu RM, Sun S, Li MW (2015) Synergetic Fe substitution and carbon connection in LiMn1–x Fe x PO4/C cathode materials for enhanced electrochemical performances. J Alloys Compd 628:471–479
Acknowledgments
This research is financially supported by the National Nature Science Foundation of China (NSFC 50902102) and Tianjin Municipal Natural Science Foundation (No. 11JCYBJC07500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, RM., Yan, SY., Sun, S. et al. Electrochemical behavior of lithium-rich layered oxide Li[Li0.23Ni0.15Mn0.62]O2 cathode material for lithium-ion battery. J Solid State Electrochem 19, 1659–1669 (2015). https://doi.org/10.1007/s10008-015-2796-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2796-9